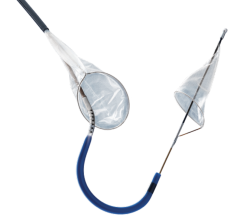
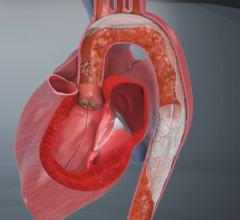
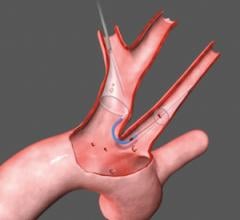
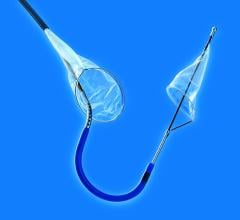
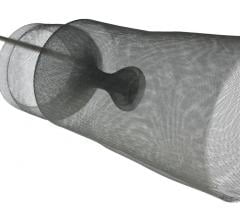
May 20, 2010 – An aortic embolic protection device, which acts as a protective shield to reduce the incidence of embolization to the brain, was granted CE mark approval this week in Europe. The Embrella Embolic Deflector will serve as an adjunctive device to be used in procedures such as transcatheter aortic valve implantation (TAVI) procedures.
This technology is currently approved in Europe for high-risk patients with severe aortic stenosis. Frequently the aorta contains atheromatous plaque and the valve can be densely calcified. During TAVI, wires, catheters and balloons pass over the aortic arch where they may dislodge debris and calcified particles. Unless deflected from the arteries leading to the head, such debris may cause a stroke.
The TAVI market is expected to grow dramatically over the next few years, so the market for the embolic protection device is expected to grow rapidly.
Embrella Cardiovascular developed the device, backed by investors that include Edwards Lifesciences Corp., maker of the Sapien transcatheter aortic valve.
For more information: www.embrella.net


 April 25, 2023
April 25, 2023