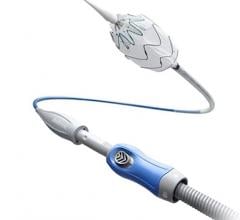
October 19, 2017 — Endologix Inc. received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to commence a confirmatory clinical study evaluating the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS) for the endovascular treatment of infrarenal abdominal aortic aneurysms. The EVAS2 IDE Multicenter Safety and Effectiveness Confirmatory Study (EVAS2) will prospectively evaluate the refined Indications for Use (IFU) and the Nellix Gen2 EVAS System.
The study is approved to enroll up to 90 primary patients, with one-year follow-up data required for the pre-market approval (PMA) application (PMA).
The Nellix EVAS system is an endovascular abdominal aortic aneurysm (AAA) therapy designed to seal the entire aneurysm. Nellix is the first and only EVAS product developed as an alternative treatment approach to traditional endovascular aortic repair (EVAR) devices, according to Endologix.
For more information: www.endologix.com


 September 18, 2025
September 18, 2025