June 16, 2023 — Esperion presented results from an analysis comparing the cardiovascular risk reduction benefits of bempedoic acid treatment with statin therapy per unit decrease in LDL-C, as observed in the CLEAR Outcomes study, at the 2023 Endocrine Society Meeting (ENDO 2023), held in Chicago, IL June 15-18, 2023.
“We are pleased to present this analysis at ENDO 2023, demonstrating that the cardiovascular risk reduction benefit of bempedoic acid treatment is comparable to that of statins based on an analysis of per unit decrease in LDL-C,” said Sheldon Koenig, President and CEO of Esperion. “As highlighted in our New England Journal of Medicine publication of CLEAR Outcomes earlier this year, the benefit of bempedoic acid treatment is consistent with the event reduction predicted by the CTT analysis, and generally improved over time, similar to what was observed in statin outcomes trials, with greater major adverse cardiovascular events (MACE-4) benefit seen in those patients who remain on the study drug for the duration of the study. Patients unable to tolerate statins or achieve their LDL-C goals on statins alone represent approximately one-third of all primary and secondary prevention patients, making the significant benefits of bempedoic acid in this patient population both evident and important.”
Key Highlights from the Analysis:
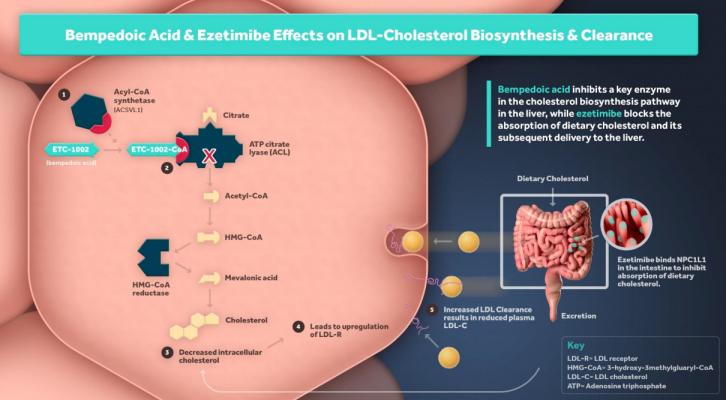
The analysis included results from all 13,970 statin-intolerant patients from the CLEAR Outcomes trial with or at high risk for cardiovascular (CV) disease and with an LDL-C level of at least 2.59 mmol/L (100 mg/dL). Using the CTT methodology, which aims to provide reliable information about the effects on mortality and morbidity of treatments that modify blood lipid levels for a wide range of patient populations and risk groups, the analysis showed that with statins, for every 1 mmol/L (39 mg/dL) reduction in LDL-C observed at 12 months, there was an associated 22% reduction in the major vascular events (MVE) endpoint, defined as the first occurrence of any major coronary event (including coronary death or non-fatal myocardial infarction), coronary revascularization, or stroke. The primary endpoint in CLEAR Outcomes was a composite of MACE-4 that included death from CV causes, non-fatal myocardial infarction, non-fatal stroke, and coronary revascularization, thus the methodology used in the analysis of statin Cardiovascular Outcomes Trials (CVOTs) was used to determine whether bempedoic acid provides similar cardiovascular risk reduction as statins, per unit decrease in LDL-C.
Results from the CLEAR Outcomes:
- Bempedoic acid was associated with:
- a 21% decrease in LDL-C at 6 months compared with placebo;
- a 23% reduction in the risk of in the composite of fatal and nonfatal myocardial infarction; (hazard ratio 0.77; 95% CI 0.66-0.91; p=0.002)
- a 19% reduction in the risk of coronary revascularization; (hazard ratio 0.81; 95% CI 0.72-0.92; p=0.001)
- a 15% reduction in the risk of the MACE-3 composite of death from CV causes, non-fatal myocardial infarction, and non-fatal stroke; (hazard ratio 0.85; 95% CI 0.76-0.96; p=0.006) and
- a 13% reduction (hazard ratio 0.87; 95%CI 0.79-0.96; p=0.0037) in the MACE-4 composite of major adverse cardiovascular events that included death from CV causes, non-fatal myocardial infarction, non-fatal stroke, and coronary revascularization in the intention to treat analysis (all randomized patients)
- a 20% reduction (hazard ratio 0.80; 95%CI 0.72-0.89; p=0.0001) in the MACE-4 composite of major adverse cardiovascular events in the analysis of patients receiving the study drug
- Patients randomized to treatment with bempedoic acid achieved a placebo-corrected lowering of LDL-C of 0.58 mmol/L (22.43 mg/dL) at month 12, and an associated 15% reduction [hazard ratio 0.85 (95%CI 0.77, 0.94)] in the risk of the CTT MVE endpoint.
- When normalized to a 1.0 mmol/L LDL-C reduction as in the CTT, the CV risk reduction with bempedoic acid shown via the CTT MVE endpoint is comparable to the normalized risk reduction with statins observed in the CTT meta-analyses of 22% [risk ratio 0.78, 95% CI 0.76, 0.80]. The beneficial effect of bempedoic acid on MVE reduction generally improved over time, similar to what was observed in statin CVOTs.
Indication
Bempedoic acid is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C. Limitations of Use: The effect of bempedoic acid on cardiovascular morbidity and mortality has not been determined.
Important Safety Information
Warnings and Precautions: Hyperuricemia: Bempedoic acid may increase blood uric acid levels. Hyperuricemia may occur early in treatment and persist throughout treatment, and may lead to the development of gout, especially in patients with a history of gout. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
Tendon Rupture: Bempedoic acid is associated with an increased risk of tendon rupture or injury. In clinical trials, tendon rupture occurred in 0.5% of patients treated with bempedoic acid versus 0% of patients treated with placebo, and involved the rotator cuff (the shoulder), biceps tendon, or Achilles tendon. Tendon rupture occurred within weeks to months of starting bempedoic acid. Tendon rupture may occur more frequently in patients over 60 years of age, patients taking corticosteroid or fluoroquinolone drugs, patients with renal failure, and patients with previous tendon disorders. Discontinue bempedoic acid at the first sign of tendon rupture. Avoid bempedoic acid in patients who have a history of tendon disorders or tendon rupture.
Adverse Reactions: In clinical trials, the most commonly reported adverse reactions were upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes. Reactions reported less frequently, but still more often than with placebo, included benign prostatic hyperplasia and atrial fibrillation.
Drug Interactions: Simvastatin and Pravastatin: Concomitant use results in increased concentrations and increased risk of simvastatin or pravastatin-related myopathy. Use with greater than 20 mg of simvastatin or 40 mg of pravastatin should be avoided.
Lactation and Pregnancy: It is not recommended that bempedoic acid be taken during breastfeeding. Discontinue bempedoic acid when pregnancy is recognized, unless the benefits of therapy outweigh the potential risks to the fetus. Based on the mechanism of action, bempedoic acid may cause fetal harm.
Please see full Prescribing Information here.
CLEAR Cardiovascular Outcomes Trial
CLEAR Outcomes is part of the CLEAR clinical research program for NEXLETOL (bempedoic acid) Tablet and NEXLIZET (bempedoic acid and ezetimibe) Tablet. The CLEAR Program seeks to generate important clinical evidence on the safety and efficacy of bempedoic acid, a first in a class ATP citrate lyase inhibitor contained in NEXLETOL and NEXLIZET and its potential role in addressing additional critical unmet medical needs. More than 60,000 people will have participated in the program by the time of its completion. The CLEAR Program includes 5 label-enabling Phase III studies as well as other key Phase IV studies with the potential to reach more than 70 million people with or at risk for CVD based on elevated LDL-C.
For more information: www.esperion.com
Related CLEAR content:


 January 28, 2026
January 28, 2026 









