September 22, 2010 - A new intravascular imaging system will be highlighted at the annual Transcatheter Cardiovascular Therapeutics (TCT) meeting in Washington, D.C. The C7-XR optical coherence tomography (OCT) platform, made by St. Jude Medical, offers physicians a new tool for intravascular lesion assessment.
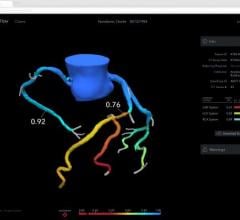
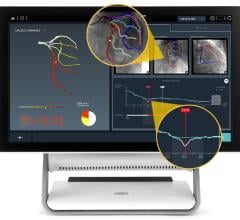
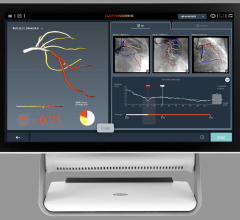
The system uses near-infrared light to create high-resolution images, allowing physicians to visualize and measure important vessel characteristics that are otherwise invisible or difficult to assess. While fractional flow reserve (FFR) accurately identifies which lesions to stent, OCT-guided treatment can be especially important for assessing stent placement because it shows precisely how the stent is positioned against the artery wall.
In addition to guiding stent selection, the C7-XR System provides physicians with post-stenting information by evaluating the position and deployment of the stent. At follow-up, the technology provides detailed information regarding the inner lining of the vessel and whether there is a reoccurrence of the blood vessel narrowing.
Physicians can learn more about OCT-guided therapy by participating in virtual reality simulations. The simulator enables physicians to manipulate the C7-XR Dragonfly catheter, performing OCT-guided procedures in human-size models and selecting from a variety of case scenarios. The sessions will take place on Sept. 23 and Sept. 24 from 9 a.m. to 5 p.m. and on Sept. 25 from 9 a.m. to 2 p.m. at the St. Jude Medical booth.
St. Jude Medical will also sponsor a scientific symposium entitled, "OCT & FFR, Current Applications, Future Potential." The symposium will take place on Thursday, Sept. 23 from 7 p.m. to 9:30 p.m. in the Renaissance Ballroom, located in the Renaissance Washington, D.C. Downtown Hotel.
For more information: www.sjm.com


 February 03, 2026
February 03, 2026