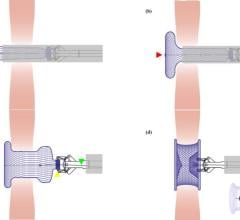
October 9, 2007 - W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder with a modified catheter delivery system indicated for the transcatheter closure of atrial septal defect (ASD), providing a percutaneous ASD closure solution for very young patients.
The GORE HELEX Septal Occluder is a permanently implanted prosthesis that uses ePTFE, a biocompatible material that allows tissue ingrowth, to seal the defect. The recently approved catheter-based delivery system allows is designed for easier device deployment via standard femoral venous access, bringing the GORE HELEX Septal Occluder to the forefront of non-surgical ASD repair.
An ASD is an abnormal hole in the wall between the upper chambers of the heart, which allows blood to improperly flow from the left side of the heart to the right, forcing the right side of the heart and lungs to overexert to compensate for the problem. Left untreated, an ASD can cause the heart to enlarge, or weaken, leaving the patient at risk for serious conditions like atrial fibrillation, pulmonary hypertension, heart failure or stroke. The defect is most often treated in pediatric patients.
The GORE HELEX Septal Occluder is composed of ePTFE patch material supported by a single nitinol wire frame that bridges and is said to eventually occlude the septal defect to stop the shunting of blood between the atria. Over the course of several weeks to months, cells begin to infiltrate and grow over the ePTFE membrane, resulting in successful closure of the defect.
"Open heart surgery is no longer the only available option to correct an ASD in young children and in patients with complicating health factors. Interventional cardiologists can close the defect permanently through a minimally invasive procedure with a shorter recovery time," said Peter Zeller, Ph.D., Gore Product Specialist.
For more information: www.goremedical.com/helex


 June 20, 2024
June 20, 2024