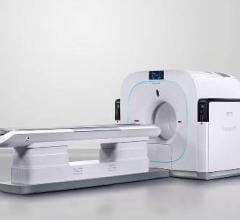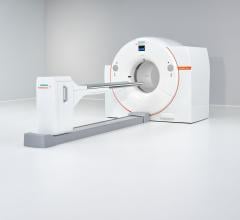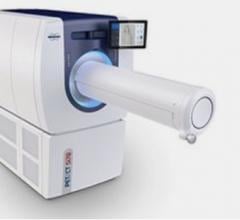
May 8, 2009 – Siemens Healthcare announces FDA 510(k) clearance for the new Biograph TruePoint 16-slice PET/CT system, which is now commercially available.
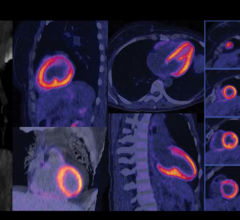
With the latest addition to the Biograph TruePoint PET/CT family, Siemens is making PET/CT technology accessible to all facilities. It features high-performance capabilities, such as High-Definition PET and routine 10-minute, whole-body imaging. In addition, the Biograph TruePoint 16 is able to provide 2 mm uniform PET resolution throughout the field of view and a two-time improvement in signal-to-noise ratio, which is designed to enable small lesion detection. The system is also available with Siemens TrueV extended PET field of view technology, which enables clinicians to perform imaging studies at twice the scan speed or half the patient dose.
The new Biograph 16-slice PET/CT system is built for patient comfort and features a 500-pound (227 kg) capacity patient bed, a 70-centimeter patient bore and efficient workflow system. The system is also mobile-ready, equipped to maximize on-location patient throughput with fast, 10-minute imaging.
The Biograph TruePoint 16-slice PET/CT system has been FDA-cleared under the name Siemens Medical Solutions USA Inc.
For more information: www.siemens.com/healthcare

 June 23, 2025
June 23, 2025