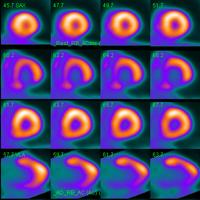
March 11, 2011 – U.S. Food and Drug Administration (FDA) and Lantheus Medical Imaging Inc. today reached agreement on a phase 3 clinical trial design to assess myocardial perfusion using flurpiridaz F18 positron emission tomography (PET) imaging in patients with suspected or known coronary artery disease (CAD).
The company and the FDA worked out a special protocol assessment for the trial and the planned analysis of the data. The company plans to initiate the first of two planned phase 3 trials in the second quarter of 2011.
“The SPA agreement is a significant milestone in the development of flurpiridaz F18 and provides us with a clearly defined path forward for the phase 3 program,” said Don Kiepert, president and CEO of Lantheus Medical Imaging. “We thank the FDA for their timely review and approval of this SPA and look forward to initiating the phase 3 program and building on the clinical trial results to date for this agent. We believe that flurpiridaz F18 can improve the diagnosis and evaluation of coronary artery disease, ultimately reducing the need for additional medical tests and procedures.”
Flurpiridaz F18 has completed a phase 2 clinical trial, and analysis of the full data set will be presented at the Nuclear Cardiology and Cardiac CT Conference, scheduled May 15-18 in Amsterdam. Preliminary phase 2 data were presented at the annual meeting of the Society of Nuclear Medicine (SNM) in June 2010. These data showed that PET imaging with flurpiridaz F18 provided better image quality than technetium-99m sestamibi single photon emission computed tomography (SPECT), the current standard for the noninvasive detection of CAD. No serious adverse events attributable to flurpiridaz F18 injection were reported in phase 1 or phase 2 clinical trials. Numerous other abstracts based on single-center evaluation of flurpiridaz F18 data were presented at various medical conferences in 2010.
“The results of the phase 2 study with flurpiridaz F18 are promising and we look forward to presenting the full data analysis later this year and initiating the phase 3 program,” said Dana S. Washburn, M.D., vice president, clinical development and medical affairs at Lantheus. “Our phase 3 program demonstrates our ongoing commitment to developing first-in-class imaging tools to advance patient care, and we remain dedicated to investigating the potential of PET technology for evaluating cardiovascular disease.”
The phase 3 clinical development program will include two open-label trials designed to assess myocardial perfusion using PET imaging of flurpiridaz F18 in approximately 1,350 patients with suspected or known CAD at approximately 100 clinical trial sites, including locations in the United States, Canada, Europe and South America. The primary objective of the study will be to assess the diagnostic efficacy (sensitivity and specificity) of flurpiridaz F18 injection PET myocardial perfusion imaging (MPI), compared with SPECT MPI in the detection of significant coronary artery disease.
A SPA is an agreement between the sponsor and the FDA indicating that the sponsor’s proposed trial protocol, including clinical endpoints and statistical analyses, are acceptable to support regulatory approval of the treatment being evaluated. FDA approval for the product is dependent on efficacy results, adverse event profiles and an evaluation of the benefit/risk of a treatment as demonstrated in the clinical trials
Flurpiridaz F18 Injection
Flurpiridaz F18 injection, a fluorine 18-labeled agent that binds to mitochondrial complex 1 (MC-1), is designed to be a new myocardial perfusion PET imaging agent for the diagnosis of coronary artery disease (CAD). PET imaging with flurpiridaz F18 has the potential to be a new clinical tool for the evaluation of myocardial perfusion that may better evaluate patients with known or suspected CAD. CAD is the most common form of heart disease, affecting approximately 16.8 million people in the United States.
For more information: www.lantheus.com

 November 17, 2025
November 17, 2025 





![Phase III clinical trial of [18F]flurpiridaz PET diagnostic radiopharmaceutical meets co-primary endpoints for detecting Coronary Artery Disease (CAD)](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-09-13%20at%203.30.13%20PM.png?itok=2w6OoNd6)
