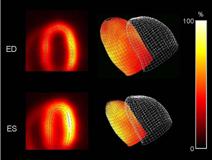
April 2, 2010 – The FDA, Society of Nuclear Medicine (SNM) and the Radiological Society of North America (RSNA) are hosting a joint two–topic workshop, April 13–14, at the Natcher Conference Center of the National Institutes of Health, Bethesda, Md.
The first day of the workshop will focus on general issues of standardization to control variability and inconsistency in methods of acquisition, interpretation and analysis of images in clinical trials. The second day of the workshop consists of an interactive tutorial on ways to address the FDA regulatory expectations for positron emission tomography (PET) drugs, particularly with respect to the recently issued regulations establishing the current good manufacturing practice (cGMP).
"This workshop offers a unique opportunity to work with the imaging community to help optimize the role of imaging in public health," said Dwaine Rieves, M.D., director of the division of medical imaging products in the FDA’s Center for Drug Evaluation and Research. "This is an example of the FDA’s focus on transparency and its collaboration with stakeholders to ensure that regulated products are both safe and effective."
"We are delighted to be partnering with the FDA and RSNA to bring the molecular imaging community together on the important transition to the new regulations," said Michael M. Graham, Ph.D., M.D., president of SNM. "The PET community remains very focused on preparing to comply with these regulations and is committed to working together to ensure a smooth transition."
"We are very pleased to be working with the FDA and SNM – and the wider community – to discuss critical issues central to the use of imaging as an endpoint in clinical trials," said Daniel Sullivan, M.D., RSNA science advisor. "Standardization is essential to improving the value and practicality of imaging biomarkers."
The FDA published a final regulation on cGMP for the production of PET drugs in December 2009. The new regulations, known as 21 CFR Part 212, take effect on Dec. 12, 2011. All PET drug manufacturers will be required to submit a new or abbreviated drug application for PET drugs in commercial/clinical use by that date. In the interim, U.S. facilities must continue to comply with USP General Chapter 823, which sets standards for the production of PET drugs.
The workshop is free and open to the public. However, early registration is encouraged, as space is limited.
For more information: www.rsna.org/snm/index.html

 November 17, 2025
November 17, 2025 





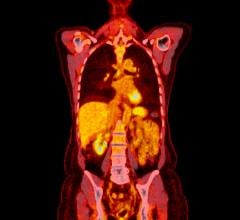
![Phase III clinical trial of [18F]flurpiridaz PET diagnostic radiopharmaceutical meets co-primary endpoints for detecting Coronary Artery Disease (CAD)](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-09-13%20at%203.30.13%20PM.png?itok=2w6OoNd6)
