January 11, 2013 — Guided Interventions LLC, a startup company developing new, easier to use fractional flow reserve (FFR) a product to help cardiologists better assess the physiological impact of coronary artery blockages, has received a $250,000 investment commitment from nonprofit venture development organization JumpStart Inc.
Guided Interventions' novel FFR guide wire provides an easier, more effective and more affordable way to obtain FFR measurements during coronary catheterization procedures.
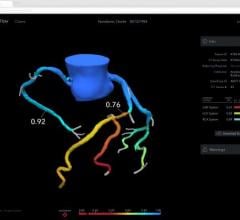
"An FFR measurement determines the best way to treat a coronary artery blockage - whether that entails no intervention, angioplasty or angioplasty plus stenting," said Guided Interventions' CEO, Matthew Pollman.
Using FFR measurements improves the precision of these diagnoses, potentially reducing unnecessary procedures and lowering medical costs while improving patient outcomes. "As these measurements increasingly become standard practice, we believe our user-friendly FFR guide wire will be adopted widely," Pollman said.
FFR guide wires currently available to cardiologists can be difficult to guide through an artery because their powered sensors require significant structural compromises to the interior core of a guide wire. Utilizing a passive sensor, Guided Interventions' novel guide wire solution is optimized for improved maneuverability and handling.
"FFR is a relatively new and somewhat under-utilized diagnostic technology," said Mike Lang, JumpStart's venture partner. "But thanks to a great product design and a strong management team with extensive experience in medical device development and commercialization, Guided Interventions is in a good position to enter and even lead the growing FFR guide wire market."
Guided Interventions plans to use the JumpStart investment to complete development and test an initial prototype in relevant animal models. The company also intends to launch and scale its operations in Northeast Ohio within calendar year 2013, especially by hiring engineers. "Northeast Ohio's resources - including Global Cardiovascular Innovation Center, the SMART Commercialization Center and North Coast Angel Fund - are first-rate," Pollman says. "Their presence makes the region an appealing place to found and grow our company, and gives us the ability to get traction in the medical device space."
For more information: www.jumpstartinc.org


 February 03, 2026
February 03, 2026