March 31, 2008 - A device that catches bits of plaque and blood clot that break loose during percutaneous coronary intervention (PCI) has failed to show that it can reduce rates of major cardiovascular complications in patients with acute coronary syndromes, a condition that encompasses unstable angina and a type of heart attack known as non-ST-elevation myocardial infarction (NSTEMI). Although the EZ FilterWire captured debris in the bloodstream in nearly half of patients, it did not reduce damage to the heart muscle.
Investigators for the Angioplasty Balloon-Associated Coronary Debris and the EZ FilterWire (A-F) study had expected the results to be more encouraging. First, the study focused on patients with arterial blockages that appeared particularly likely to be a source of downstream debris during PCI. In addition, myonecrosis, or debris-caused damage to the heart muscle resulting from blockage of tiny blood vessels, is fairly common.
“PCI is associated with myonecrosis in about 25 percent of patients with non-STEMI acute coronary syndromes,” said Mark W.I. Webster, MD, director of the cardiac catheterization laboratory at Auckland City Hospital, Auckland, New Zealand. “Although there are other mechanisms, distal embolism of atherosclerotic plaque and/or thrombus is recognized to be a frequent cause.”
The A-F study is being presented today in a Late-Breaking Clinical Trials session at the SCAI Annual Scientific Sessions in Partnership with ACC i2 Summit (SCAI-ACCi2) in Chicago. SCAI-ACCi2 is a scientific meeting for practicing cardiovascular interventionalists sponsored by the Society for Cardiovascular Angiography and Interventions (SCAI) in partnership with the American College of Cardiology (ACC).
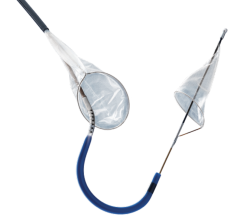
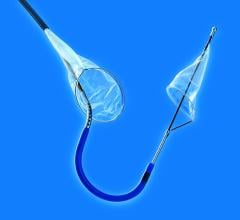
The EZ FilterWire is a coronary guidewire with a plastic sack shaped like a windsock attached. The windsock has multiple small holes to allow blood to flow through, but it catches larger debris. During PCI, the FilterWire is positioned in the coronary artery downstream of the lesion; both the device and any debris are removed after the procedure.
The A-F trial is the first randomized controlled trial to evaluate a vascular protection device in patients with NSTEMI acute coronary syndromes. For the study, Dr. Webster and his colleagues recruited 151 patients treated at 14 medical centers in New Zealand, Australia and Canada, randomly assigning them to protection with the FilterWire or to conventional PCI without vascular protection. The researchers found that the rates of major cardiovascular complications during hospitalization—consisting of death, heart attack, emergency bypass surgery, or repeat procedure in the treated artery—were no different in the two groups (12 percent in the FilterWire group vs. 10 percent in the control group). There were also no significant differences between the groups in the post-procedure release of enzymes that signal damage to the heart muscle.
“The device does not appear warranted for routine use in these patients,” Dr. Webster said. “However, there are some patients who have major complications from distal embolism. We need better ways of identifying high-risk patients and lesions.”
Dr. Webster will present the results of the "Angioplasty Balloon-Associated Coronary Debris and the EZ FilterWire” (A-F) study on Sunday, March 30 at 8:00 a.m. CDT in the Grand Ballroom, S100.
For more information: www.acc08.org


 April 25, 2023
April 25, 2023