January 8, 2018 — W. L. Gore & Associates Inc. announced the first implant of the Gore Excluder Conformable AAA (abdominal aortic aneurysm) Endoprosthesis in the United States. The successful procedure took place on Dec. 19, 2017, at Maimonides Medical Center in New York by Robert Rhee, M.D., chief of vascular and endovascular surgery, and national principal investigator.
This endovascular aortic repair (EVAR) device, which is the first to feature angulation control, is part of an investigational clinical study approved by the U.S. Food and Drug Administration (FDA). The clinical study will assess the safety and effectiveness of the device in treating infrarenal AAAs in patients with challenging anatomy. The clinical study consists of two sub-studies, each assessing the device for a different range of patient anatomies. The implantation by Rhee is part of the short neck sub-study to assess the device in aortic neck angles of 0 to 60 degrees and aortic neck lengths of 10 mm or greater. The high neck angulation sub-study will evaluate proximal aortic neck angles of 61 to 90 degrees and aortic neck lengths of 10 mm or greater.
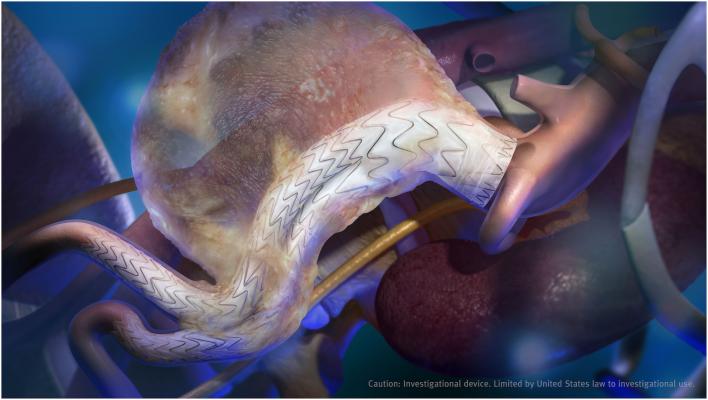
“Patients with extreme proximal neck anatomies often do not qualify for EVAR. Regardless of device flexibility, current delivery systems make it difficult to achieve conformability during deployment,” said Rhee. “The angulation control in the new delivery system for the Gore Excluder Conformable AAA Device is intended to allow physicians a greater level of control to angle or bend the device to achieve orthogonal placement to the aortic blood flow lumen.”
The Gore Excluder Conformable AAA Device builds on the clinical performance of the Gore Excluder Device. The new device leverages the limb design of the first-generation device which has demonstrated exceptional clinical performance as evidenced by 0.5 percent limb occlusion through three-year follow-up. The limbs — a combination of proprietary ePTFE graft material and a fully supported, nested nitinol stent — are designed to prevent kinking and occlusion.
The Gore Excluder Conformable AAA Device debuts the Gore Active Control System into the abdominal aorta. This delivery system includes angulation control, giving physicians the option to angle or bend the device to achieve orthogonal placement to the aortic blood flow lumen and to maximize the conformability and seal of the device. Like the Gore C3 Delivery System, the Gore Active Control System offers the ability to reposition the device if needed after initial deployment to achieve optimal device placement.
For more information: www.gore.com
Related Content
Gore Announces First-in-Human Use of Gore TAG Conformable Thoracic Stent Graft
VIDEO: How to Implant a Dialysis Access Graft in a Complex Patient


 January 05, 2026
January 05, 2026 









