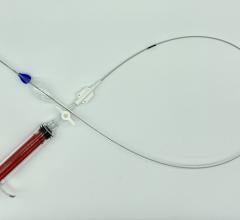
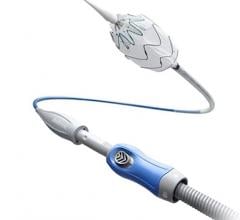
July 17, 2017 — W. L. Gore & Associates Inc. announced the first patient implant of the Gore TAG Conformable Thoracic Stent Graft with Active Control System after receiving CE Mark last month. The first implant was performed by Prof. Dr. med. Giovanni Torsello and Dr. med. Martin Austermann at St. Franziskus Hospital, Munster, Germany.
The thoracic endovascular aortic repair (TEVAR) device is the first to feature a new delivery system that provides the physician with controlled, staged deployment, according to the company. The system optimizes accuracy, angulation and apposition to treat etiologies of the descending thoracic aorta including aneurysms, transections, and acute and chronic Type B dissections. The new device will be formally launched in European regions later this year.
The Gore Active Control System enhances the conformability of the stent graft, facilitating the optimized wall apposition of the Conformable Gore TAG Device even in complex anatomies, such as acute aortic angles. The novel staged deployment feature enables the physician to refine positioning and angulate the stent graft within the body to achieve optimal placement prior to full-diameter expansion. The angulation control capability gives physicians the option to angulate the device to achieve orthogonal placement to the aortic blood flow lumen and maximize conformability and seal. These features enable physicians to more confidently perform endovascular treatment even in anatomies where tortuous vessels might historically have suggested open surgery.
“The enhanced control capabilities this new delivery system allows could reduce many common complications that can occur if the stent graft is not placed correctly during the TEVAR procedure,” commented Torsello. “Secondary interventions can be traumatic for patients and costly to the provider. The innovative staged deployment feature of the Gore Active Control System provides a level of precision that has never existed in TEVAR. This level of control in combination with the long-term benefits of the stent graft is a significant advancement for the field.”
The new product offering features the same stent graft as the Conformable Gore TAG Device, whose outcomes have been established through its long-term freedom from reintervention (89 percent) and low serious endoleak (6 percent) and migration (0 percent) rates through five years, according to the TAG 08-03 clinical trial for treatment of aneurysms of the descending aorta. The device is a combination of proprietary ePTFE graft material and a fully supported, nested, nitinol stent.
For more information: www.gore.com


 September 18, 2025
September 18, 2025