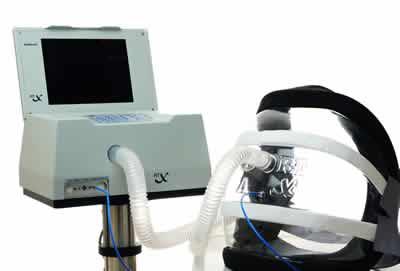
Image courtesy of Hayek Medical
May 2, 2018 — Select adult patients born with a single functioning ventricle, and who have undergone a surgical operation called the ‘Fontan procedure’ during childhood, are being enrolled in a new global-first clinical trial. The trial, led by a multi-disciplinary team of heart and lung physicians, will examine the effects of a portable, non-invasive medical device never before tested on patients with this cardiac condition.
“As the exclusive global site for this important device therapy study, our investigators will be able to select specific complex congenital heart disease patients from the largest cohort of Fontan repair survivors in the world,” said Barry Rubin, M.D., medical director, Peter Munk Cardiac Centre (PMCC), University Health Network in Toronto, Canada. “This trial, funded in part by our PMCC Innovation Committee, provides access to suitable patients as well as in-house clinical trials expertise, equipping our medical team with the critical tools necessary to potentially unravel a decades-old medical challenge in congenital cardiology — supporting those patients whose survival into adulthood is often accompanied by a poor quality of life, often leading to sudden death.”
The study entitled, FONTAN-CMR (Feasibility and efficacy Of Negative Pressure Ventilation in The Ambulatory populatioN-Cardiovascular flow assessment by Magnetic Resonance imaging), will test a portable ventilation system to assist breathing and augment the circulation of a Fontan patient living with only one effective pumping chamber. This study is also supported by the Labatt Family Heart Centre Innovation Fund at SickKids Hospital.
“Adults with a Fontan circulation are at high-risk of cardiac complications which can be caused by the body’s adaptive response to decades of increased pressures in the Fontan circuit,” said Rachel Wald, M.D., adult congenital and pediatric cardiologist, Peter Munk Cardiac Centre and co-principal investigator of the study. “Short of cardiac transplantation, which can be accomplished in relatively few patients, we are left with extremely limited therapies to offer patients when complications begin as a result of failing Fontan physiology. It is our hope that this device will effectively enhance cardiac output, and in doing so, might one day be considered a future bridge to cardiac transplantation or perhaps even destination therapy.”
“Patients with ‘failing Fontan physiology’ will present with fatigue, decreased exercise tolerance, rhythm abnormalities and accumulation of fluid in their limbs and/or abdomen,” said Pradeepkumar Charla, M.D., adult congenital cardiology fellow, Toronto General Hospital, and co-principal investigator of the study. “A chronic, low cardiac output state coupled with increased venous congestion often results in these patients developing multi-organ dysfunction which typically affects the liver and kidneys,” he said.
“The Hayek RTX uses Biphasic Cuirass Ventilation (BCV) to provide an efficient and effective method of non-invasive external ventilation. BCV mimics the natural physiology of spontaneous ventilation with an active expiratory phase,” said Joseph Cronin, M.D., medical director, United Hayek Medical, manufacturer of the Hayek RTX. He said that the ventilator operates via an external cuirass, or shield, which can be worn by patients while awake or asleep, in or out of the hospital.
The initial phase of FONTAN-CMR is designed to explore the safety and efficacy of the portable ventilator to improve cardiac output in adult Fontan survivors using CMR to precisely measure flows into and out of the heart. Study participants have been selected from more than 350 Fontan patients in the adult congenital heart program at PMCC, which has a database of more than 9,000 actively followed adult patients who were born with a heart defect. Phases two and three of the trial will allow children and adults to wear this device for longer periods of time while at home.
For more information: www.petermunkcardiaccentre.ca


 January 05, 2026
January 05, 2026 









