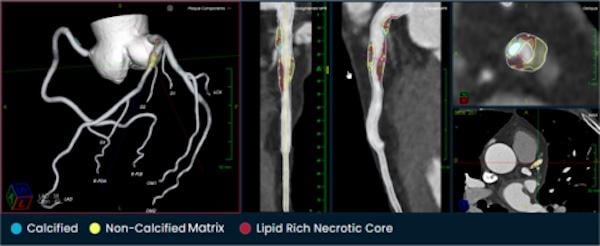
PHOTO CAPTION: The Elucid PlaqueIQ user interface is a fully interactive visualization of the patient’s coronary anatomy, showing specific plaque type and amount across various views to inform physician assessment of risk and patient-specific treatment pathway. The original CT image is preserved to provide further context.
Elucid has announced it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its PlaqueIQ imaging analysis software to help physicians diagnose cardiovascular disease (CVD).
PlaqueIQ is the first FDA-cleared non-invasive software that can objectively quantify and classify plaque morphology based on ground-truth histology, the gold standard for characterization of plaques. PlaqueIQ is designed to give physicians new, clinically validated information to help stratify patients and inform patient-specific treatment pathways.
“The fact that low-risk, asymptomatic patients represent such a large portion of the population means that even a small fraction of them account for a substantial number of myocardial infarctions (MI),” said Dr. Amir Ahmadi, Clinical Associate Professor of Medicine and Cardiology, Icahn School of Medicine at Mount Sinai, and co-director of the Cardiac Intensive Care Unit at Mount Sinai Fuster Heart Hospital at Morningside.
“It’s time to shift our focus from merely estimating risk and treating risk of MI to directly visualizing and treating the disease itself by looking at the coronary arteries,” continued Dr. Ahmadi. “I believe that PlaqueIQ will enable physicians to better ‘see’ the disease—specifically plaque quantity and type—so that we can treat patients with greater precision and in personalized manner, improve their quality of life, and ultimately prevent MI and stroke more effectively.”
PlaqueIQ uses first-line diagnostic CCTA and develops comprehensive, interactive reports to help physicians virtually “see” plaque at the vessel level. With its basis in histology, the software is uniquely able to non-invasively quantify and characterize non-calcified plaque and its components such as lipid-rich necrotic core (LRNC), giving potential insights into high-risk plaques that are key drivers of risk of heart attack and stroke.1 In addition, use of the software has the potential to enable earlier identification of higher-risk plaque before presence of symptoms or major adverse events.
“PlaqueIQ’s underpinnings in histology is a novel approach to the field of non-invasive coronary plaque classification,” said Dr. Mark Rabbat, professor of Medicine and Radiology, director of Cardiac CT, and director of Structural Heart Disease Interventional Imaging in the Division of Cardiology at Loyola University Chicago. “Armed with additional data on vulnerable plaque components, we can make more informed treatment decisions on drug therapy selection or the need to send the patient to the cath lab. I believe plaque quantification has the potential to greatly improve outcomes for patients while providing tremendous savings to the healthcare system.”
Physicians simply send patient images to Elucid with a single mouse click. Then Elucid applies PlaqueIQ’s image-restoration algorithms to the file to mitigate motion and calcium blooming artifacts. Specially trained analysts segment the data creating a 3D model of the patient’s coronary arteries. The software then identifies, classifies and quantifies tissue structure and composition.
Elucid is currently performing beta testing on PlaqueIQ and anticipates making the software available for limited release later in Q4 2024. The company is also pursuing an indication for non-invasive measurement of fractional flow reserve (FFRCT), uniquely derived from its PlaqueIQ technology, to measure coronary blockages and the extent of ischemia.
1 A. Hafiane, Vulnerable plaque, characteristics, detection, and potential therapies, J. Cardiovasc. Dev. Dis. 6 (3) (2019).


 January 15, 2026
January 15, 2026 









