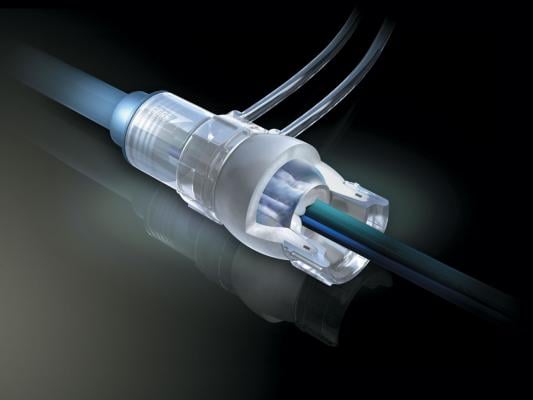
March 25, 2013 — W. L. Gore & Associates introduced its Gore DrySeal Sheath with hydrophilic coating in Europe. The new sheath allows for easier insertion into and removal from blood vessels during endovascular repair procedures. The device is designed to increase sheath lubricity with an advantage of minimizing particulation of the hydrophilic coating.
“Adding hydrophilic coating to this Gore device provides physicians with more control when performing endovascular repair,” said Thomas Larzon, M.D., head of vascular surgery, department of cardiovascular and thoracic surgery, Örebro University Hospital, Sweden. “This advantage greatly benefits patients as it helps minimize unnecessary blood loss when inserting endovascular devices.”
The sheath is comprised of the hemostatic Gore DrySeal Sheath valve attached to the introducer sheath. The Gore DrySeal Sheath valve is pressurized to create a seal, thereby minimizing blood loss and accommodating multiple wires and catheters simultaneously. The valve consists of a silicone outer tube and an inner film tube that create an effective hemostatic seal that easily adapts to the profiles of the inserted devices.
Gore received CE mark approval for the Gore DrySeal Sheath in April 2010. The device is used in conjunction with the Gore Excluder AAA endoprosthesis to aid in the minimally invasive treatment of patients with abdominal aortic aneurysms (AAA). It is also used with the conformable Gore Tag thoracic endoprosthesis in patients with isolated lesions of the descending thoracic aorta.
For more information: www.goremedical.com


 September 18, 2025
September 18, 2025 









