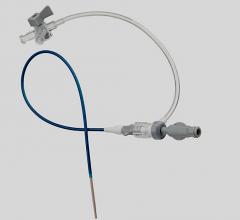
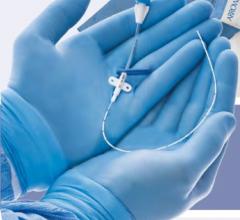
November 17, 2009 – Following three reports of stretching and fractures during procedures, the ViperSheath Sheath Introducer was voluntarily recalled on November 2 by Cardiovascular Systems Inc. (CSI).
The company said the long-coil reinforced, kink-resistant catheter sheath may fracture, separating segments that may require unplanned surgery to remove them, or to control bleeding. Any separation of the cannula has the potential to expose portions of the coil, creating the potential for vessel dissection or perforation.
CSI notified all customers by FedEx about the recall and is arranging for the return of all products. Customers with questions may contact CSI at (877) 274-0360. The recall encompasses products distributed from March 25, 2009 through Oct. 21, 2009.
CSI is working with the product manufacturer, Thomas Medical, which has apprised the U.S. Food and Drug Administration (FDA) of this action. Any adverse events with the use of this product and/or quality problems should be reported via the FDA’s MedWatch Program by fax at (800) FDA-0178, by mail at MedWatch, HF-2, FDA, 5600 Fishers Lne., Rockville, MD 20852-9787, or on the MedWatch Web site at www.fda.gov/medwatch.
For more information: www.csi360.com

 June 10, 2020
June 10, 2020