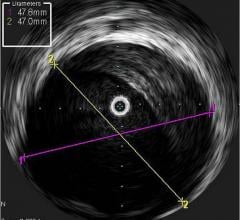
Volcano Corp., Stanford University, Cardialysis and the Cardiovascular Research Foundation in which Volcano will provide IVUS catheters free of charge for use in pivotal drug-eluting stent (DES) clinical trials. The offer will be made available to stent manufacturers utilizing the core lab services of Stanford, Cardialysis or CRF in trials where IVUS images and data are planned to be collected in 100 percent of patients at enrollment and at pre-determined follow-up periods.
The collaboration between Volcano and the highly respected core labs at Stanford, Cardialysis and CRF was orchestrated with the interventionalists guiding the core labs, Dr. Peter Fitzgerald, Dr. Patrick Serruys, Dr. Martin Leon and Dr. Gary Mintz.
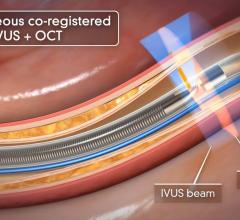
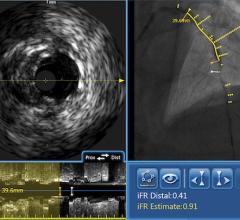
The goal of the collaboration is to provide the stent industry, physicians and patients with a more in-depth understanding of the complex phenomenon of stent thrombosis and the most definitive, expeditious answers to questions surrounding the long-term safety of current and next generation DES. In particular, IVUS is designed to precisely visualize late stent mal-apposition (when the coronary vessel grows away from the implanted stent months or years after deployment), which is emerging as a leading suspect in the search for the cause of late stent thrombosis in DES.
Comments From Collaborating Physicians:
According to Martin B. Leon, MD, Chairman of the Cardiovascular Research Foundation, and Associate Director of the Center for Interventional Vascular Therapy (CIVT) at Columbia University Medical Center, New York City, IVUS is the only imaging modality available today that provides sufficient information about the interaction between the stent and the coronary vessel.
"We have always insisted that at least a sub-group of patients receive IVUS imaging at index and follow-up. Unfortunately, the sample size has apparently not been large enough to truly understand the safety profiles of these stents. With this agreement between Volcano, CRF and the other top core labs, we will now have the information at our disposal to fully and completely characterize the performance and safety of these important, state-of-the-art DES."
Patrick Serruys, MD, head of the interventional department at Erasmus University, Heartcenter Rotterdam, The Netherlands commented:
"Prior clinical trials of DES have had important limitations in terms of the length of follow-up and the percentage of cases where follow-up IVUS images were available. As a result, we had to look to major adverse cardiac event (MACE) rates as an indication of the long-term safety of these devices. A better, more thoughtful and scientific approach is to view the IVUS images at index and follow-up in all patients in the trials. This should provide us with an acceptably large sample size upon which to determine which stents are safe, and which need additional refinement. Only in this way will we be able to understand the true safety profile of these complex DES devices."
Peter Fitzgerald, MD, PhD, Professor of Medicine and Engineering and Director of the Center for Cardiovascular Technology at Stanford University commented:
"Angiography does not allow you to visualize late stent mal-apposition. We now believe that late acquired stent mal-apposition is a key suspect in the hunt for the cause of rare, but devastating late stent thrombosis in DES. IVUS is widely available and clearly and easily visualizes late stent mal-apposition. With access to free IVUS catheters, we are now able to make a very compelling argument to all DES manufacturers that the IVUS data should be collected in 100 percent of DES study patients, even if the IVUS information isn't required to be utilized by the interventionalist when implanting the device. Without IVUS data in the vast majority of the patients, I fear that DES trials would otherwise have to become so large (in terms of number of patients enrolled) and/or have such a long duration (longer follow-up period), that the innovation in this field would come to a standstill. I applaud Volcano for its vision and commitment in working with us to put this collaboration in place."
For more information, visit www.volcano.com


 September 18, 2025
September 18, 2025