February 17, 2015 — Micell Technologies, Inc. announced the commercial availability of the MiStent sirolimus-eluting absorbable polymer coronary stent system (MiStent SES) in Europe. Stentys, Micell’s distribution partner in Paris, plans a controlled launch in Western Europe followed by a full commercial launch for the second half of 2015 in selected countries within Europe, Middle East, Southeast Asia and Latin America.
The device has received CE Mark in the European Union, and will be distributed exclusively by Stentys around the world, with the exception of the United States, Canada, China, South Korea and Japan. Micell is in the process of expanding its manufacturing capabilities through its partner, Surgical Technologies, Inc., based in St. Paul, Minn.
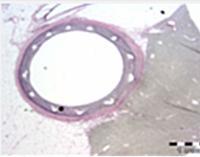
MiStent SES is designed to optimize healing in patients with coronary artery disease. The rapidly absorbable coating is intended to precisely and consistently provide for local drug delivery and limit the duration of polymer exposure, thereby potentially reducing the safety risks associated with current commercially available drug-eluting stents. The device includes a proprietary stent coating that contains crystalline drug (sirolimus) and an absorbable polymer. The coating provides controlled and sustained release of therapeutic levels of drug as the polymer disperses from the stent into the adjacent tissue. These properties are intended to enhance safety as compared to conventional permanent polymer drug-eluting stents.
EU approval of MiStent SES was supported by clinical data from two studies, DESSOLVE I and II, which demonstrated superior in-stent late lumen loss rates and a high safety profile. The three-year follow-up of the DESSOLVE clinical studies subjects was completed in 2014, and these patients continue to undergo long-term follow up.
For more information: www.micell.com


 July 02, 2024
July 02, 2024 









