April 11, 2008 - Nonin Medical Inc. this week unveiled one of the first fingertip oximeters to use Bluetooth wireless technology at the American Telemedicine Association (ATA) meeting in Seattle, WA.
The Onyx II, Model 9560 fingertip pulse oximeter is designed for interoperability, meeting the requirements of the emerging Medical Device Profile (MDP), IEEE11073 and Continua compliance standards.
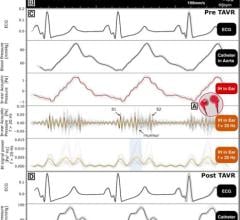
One area of healthcare that stands to benefit greatly from wireless physiological monitoring devices is chronic disease management. Patients who suffer from diseases such as chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF) or asthma will be able to regain independence using the device, the company said. The vital signs can be monitored by the patient and sent wirelessly through communication devices (cell phones, PDAs, PCs, etc.).
For more information: www.nonin.com


 October 21, 2025
October 21, 2025