April 11, 2017 — Through a new clinical trial, patients at Harrisburg, Pa.-based PinnacleHealth have access to an investigational device designed to reduce risk of stroke during transcatheter aortic valve replacement (TAVR).
Aortic stenosis is one of the most common and most serious heart valve diseases. Left untreated, aortic stenosis gradually diminishes quality of life and leads to heart failure and death. The gold standard for treating aortic stenosis has been surgical aortic valve replacement; however, changes in cardiovascular medicine have led to the minimally invasive, catheter-based TAVR procedure for patients deemed too high risk for surgery. Patients having either type of aortic valve replacement are at risk of brain injury that may range from memory loss and speech challenges to debilitating strokes.
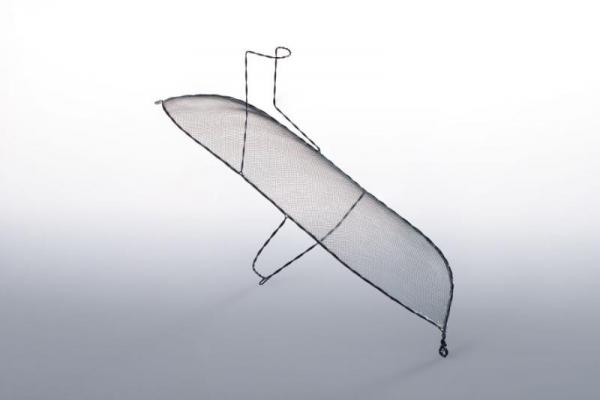
The international clinical trial, called REFLECT, studies the safety and efficacy of the Keystone Heart TriGuard cerebral embolic protection device to minimize the risk of cerebral damage during TAVR and other cardiovascular procedures. TriGuard is designed to reduce the amount of embolic debris from traveling through the blood stream to the brain for patients undergoing TAVR. If this debris travels through the blood stream to the brain, it could possibly cause damage that may lead to a stroke or neurocognitive dysfunction, such as memory loss and speech challenges.
"As physicians, we see firsthand the debilitating effects of brain injury following TAVR can have on our patients," stated Mubashir Mumtaz, M.D., FACS, FACC, chief of cardiothoracic surgery, surgical director of structural heart and principal investigator for the trial at PinnacleHealth. "PinnacleHealth CardioVascular Institute is honored to be selected for this elite global trial, studying a major patient safety initiative."
"This device presents the opportunity to minimize brain injury following TAVR and supports our goal to preserve quality of life," stated Hemal Gada, M.D., MBA, interventional cardiologist and medical director of structural heart at PinnacleHealth.
The TriGuard device is shaped to accommodate anatomic variations of the aortic arch in order to provide full coverage to all brain territories. It is placed via one of the femoral artery access ports typically used in TAVR, thereby eliminating the need for a third puncture site.
PinnacleHealth is one of 27 hospital systems in the world, and the only hospital in Pennsylvania, enrolling patients in this trial.
For more information: www.keystoneheart.com


 November 14, 2025
November 14, 2025 









