February 9, 2022 — A new study has shown how SARS-CoV-2 may contribute to severe microvascular damage seen in severely-ill COVID-19 patients by transforming human heart vascular cells into inflammatory cells, without infecting them. The University of Bristol-led research, published in Clinical Science, indicates blocking antibodies could represent a new treatment to alleviate cardiovascular complications.
In this new study, a multidisciplinary research team from the University’s Bristol Heart Institute sought to investigate how SARS-CoV-2 interacts with heart cells causing the myocardial damage seen in COVID-19 patients. Until now, it remained unclear whether heart cells are infected by the virus or damaged because of an excess cytotoxic defence response. This response, also known as ‘the cytokine storm’, comes from our immune cells, whereby cytotoxic cells attack and kill the infected cells by releasing proteins, called cytokines. The team also sought to investigate whether heart cells contribute to producing excess cytokines.
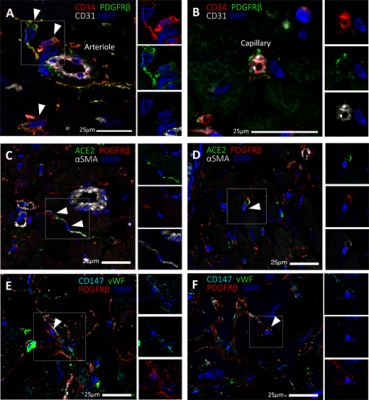
A research team led by Bristol’s Professor Paolo Madeddu exposed human heart pericytes, which are cells that wrap small blood vessels in the heart, to SARS-CoV-2 Alpha and Delta variants, along with the original Wuhan virus. Surprisingly, they found the heart pericytes were not infected.
Intrigued by this finding, in a second test-tube experiment, the researchers challenged the cardiac pericytes with the spike protein alone, without the virus. The spike protein made pericytes unable to interact with their companion endothelial cells and induced them to secrete inflammatory cytokines, suggesting the spike protein is harmful to human cardiac cells. Interestingly, the team found that antibodies blocking CD147 - a receptor for the spike protein – protected heart pericytes from damage.
Finally, the team identified the presence of the SARS-CoV-2 spike protein in blood samples obtained from COVID-19 patients, which opens the possibility that spike protein particles traveling through the circulation can reach a site distant from the respiratory system and cause systemic damage.
Dr Elisa Avolio, the study’s first author from the University’s Bristol Medical School, said: “Pericytes are essential cells of the heart, although their role in maintaining the structural integrity of the coronary vascular tree has emerged only recently. Our ongoing research on human cardiac pericytes indicates these cells co-operate with coronary endothelial cells during healing from a heart attack. This new study shows that the spike protein jeopardises this interaction and transforms pericytes into inflammatory cells. Hopefully, CD147 blocking antibodies could represent a new treatment to alleviate cardiovascular complications in COVID-19 patients.”
Professor Paolo Madeddu, cardiologist and the study lead from the University’s Bristol Medical School, added: “Microvascular complications are frequent and harmful in patients with COVID-19, with up to 11 percent of those hospitalized in intensive care units having myocardial damage or having suffered a heart attack. Furthermore, people with pre-existing cardiovascular diseases are more likely to die of COVID-19.
“Our findings newly suggest that SARS-CoV-2 can damage vascular cells without infecting them. In addition, cleaved spike protein particles could amplify the damage induced by the engagement of the full virion with vascular cells.”
“The Omicron variant has multiple mutations to its spike protein, which helps the virus to enter and infect human cells, resulting in higher transmissibility and stronger binding with human cells.”
“However, in the case of the current Omicron wave, experts say there haven't been any cardiac symptoms reported so far although it is still very early to say for sure. If confirmed, this may indicate a dissociation between infectivity and capacity of SARS-CoV-2 to cause cardiac cell damage. The multifunctional spike protein being the key determinant in these phenomena.”
The research has been supported by a pump-priming grant from the Wellcome Trust, the Elizabeth Blackwell Institute (EBI) Rapid Response COVID-19 and a British Heart Foundation grant (number PG/20/10285). The authors are members of the University of Bristol COVID19 Emergency Research Group (UNCOVER).
For more information: www.bristol.ac.uk/research/impact/coronavirus/research-priorities/
Related COVID-19 Cardiology Content:
The Long-term Cardiovascular Impact of COVID-19
COVID-19 Changes Properties Blood Cells
VIDEO: Antithrombotic Prophylaxis in COVID-19 Patients — Interview with Behnood Bikdeli M.D.
Coronavirus Disease 2019 (COVID-19) and the Heart—Is Heart Failure the Next Chapter?
PHOTO GALLERY: How COVID-19 Appears on Medical Imaging
Heart Damage Found in More Than Half of COVID-19 Patients Discharged From Hospitals
Overview of Randomized Trials of Antithrombotic Therapy for COVID-19 Patients
COVID-19 Can Kill Heart Cells and Interfere With Contraction

 March 20, 2024
March 20, 2024