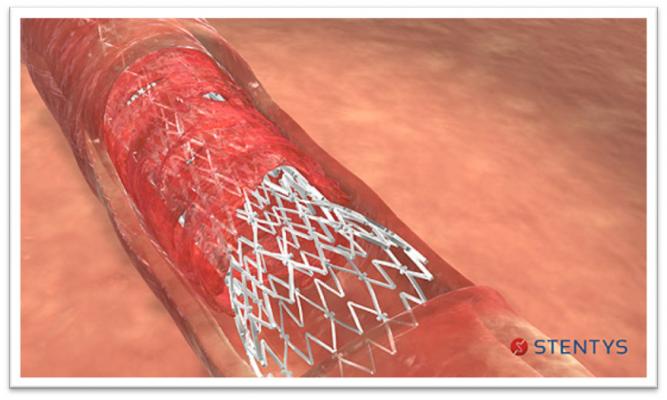
Xposition S image courtesy of Stentys
June 17, 2015 - Stentys announced that the findings of the first Xposition clinical experience, as part of the SETUP trial, were presented at the EuroPCR conference in May. The results of this study were also published in the online edition of EuroIntervention.
Xposition S is Stentys' next-generation sirolimus-eluting self-apposing stent. It is delivered by a unique system that closely replicates conventional stent implantation techniques: A small balloon peels the sheath containing the stent to release it precisely at the intended location.
The SETUP study was a prospective, single-arm first-in-man study performed in two Dutch centers (Academic Medical Center, Amsterdam, and Albert Schweitzer Hospital, Dordrecht) to evaluate the feasibility of the use of the novel Stentys Xposition S in de novo coronary lesions. The study enrolled 25 patients and demonstrated 100 percent technical and angiographic success rates without longitudinal geographical miss on quantitative coronary angiography (QCA). Stent strut malapposition as assessed by optical coherence tomography (OCT) was very low (0.6 percent) at the end of the procedure.
Principal investigator Karel Koch, M.D., Ph.D., from AMC hospital (Amsterdam, the Netherlands), stated, "Stentys' novel balloon delivery system allowed for easy and accurate deployment of all stents at the target lesions, which of note, were performed with real-world lesion complexities, including ostial lesions, highly calcified lesions and lesions with high thrombus burden. We noticed significant improvement over the previous system and see how Xposition S could advance the quality of treatment for heart attack patients or patients with variable artery anatomy."
For more information: www.stentys.com


 July 02, 2024
July 02, 2024 









