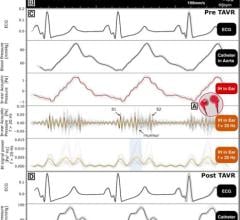
August 28, 2007 - Scivanta Medical Corp. has entered into a development agreement with Sparton Medical Systems, in which Sparton will provide Scivanta engineering and development support for the hardware component of the Hickey Cardiac Monitoring System (HCMS).
The services to be provided by Sparton include planning and development of design control documents; concept development, including mechanical, electrical and software design; completion of a detailed design and an engineering model; assembly of prototype models and preliminary design verification testing; the production of pilot devices using formal drawings and validated processes; and design verification testing on the pilot units. Sparton may bill an estimated $1.6 million for services and materials provided under the development agreement.
For more information: www.sparton.com
News | August 27, 2007


 October 21, 2025
October 21, 2025