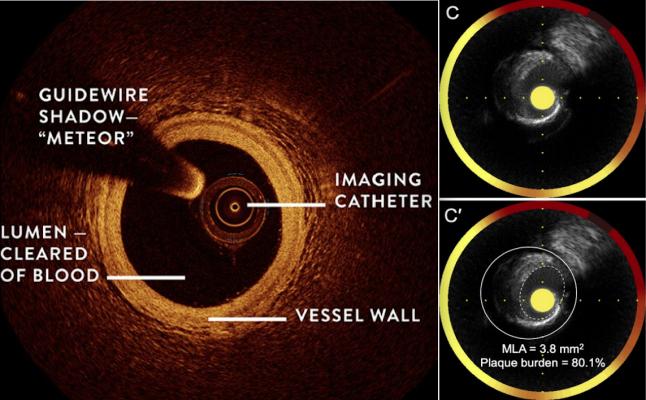
SpectraWave is developing a a new intravascular imaging system that combines two currently available modalities. This likely includes optical coherence tomography (OCT), left, which can define structures within the vessel and vessel wall. Near infrared spectroscopy (NIRS), right, enables a spectral chemical analysis of the vessel walls to show areas of high lipid content, and specifically can identify lipid-core plaques that are associated with heart attack-causing plaque ruptures.
February 16, 2021 – SpectraWave Inc. announced a $13.2 million series A-2 financing to support completion of product development and regulatory filing for its flagship intracoronary imaging system. The intravascular imaging system is designed to help physicians optimize coronary stenting interventions and limit future adverse events. The funding round is led by prior investor Deerfield Management, and includes follow-on investment from prior unnamed seed investors.
The company is developing a medical imaging platform that combines two state-of-the-art technologies providing high resolution coronary artery disease (CAD) plaque structure and to show the vessel content in coronary arteries. The imaging data will aid interventional cardiologists during stent optimization and in their assessment of the patient’s risk for future adverse events.
“This is an exciting day for the SpectraWave team who have made incredible progress developing a cost-effective, high quality, easy-to-use product for interventional cardiologists,” said Eman Namati, Ph.D., CEO of SpectraWAVE. “The continued commitment from Deerfield, in addition to the significant follow-on investments from prior investors further validates our intravascular imaging technology, and it’s potential to help patients globally.”
With the new funds, SpectraWave will continue to scale the team, development, and operations ahead of a regulatory filing. The company has recently appointed additional experienced industry talent, including leaders in image analysis, clinical affairs, and regulatory and quality affairs and established a world-renowned Clinical Advisory Board of experts.
The company was founded by Gregg Stone, M.D., Guillermo Tearney, M.D., and James Muller, M.D. The Clinical Advisory Board currently includes expert Chuck Simonton, M.D., who currently works as VP and CMO for Abiomed and previously was chief medical officer for Abbott Vascular.
Several members of the company's leadership team have extensive experience in optical coherence tomography (OCT) technology development. Tearney’s lab at Mass General also has invented a next generation OCT technology, termed µOCT (micrometer OCT), which has a resolution of 1 µm (micrometer) and is capable of imaging cells and subcellular structures in the body. A micrometer, also commonly called a micron, is equal to one millionth of a meter. For a sense of the scale, market-leading coronary stents Xience, Promus and Resolute have stent struts with a thickness of between 80-90 microns. Muller was the founder of the company InfraReDx Inc., which developed a catheter-based near-infrared spectroscopy (NIRS) system that could identify cholesterol rich plaques in patients.
“SpectraWAVE’s novel multi-modality imaging catheter aids physicians in targeted coronary interventions and has the opportunity to make a fundamental impact on the lives of coronary artery disease patients,” said Steve Hochberg, Partner at Deerfield Management, lead Series A-2 investor. “The complexity of mid-procedure visualization and its impact on clinical decision making requires a truly unique product and a talented team to bring it to market. SpectraWAVE has shown immense progress, and we’re pleased to continue our support as it rapidly moves towards its first regulatory filing.”
For more information: www.spectrawave.com


 November 14, 2025
November 14, 2025 









