July 28, 2010 – This week St. Jude Medical filed a patent infringement lawsuit against Volcano Corp. over its fractional flow reserve (FFR) wires. The suit was filed in federal district court in Delaware. Volcano said the claims against it are entirely without merit.
The complaint alleges Volcano infringed on five St. Jude patents related to its PressureWire. St. Jude Medical seeks injunctive relief and monetary damages.
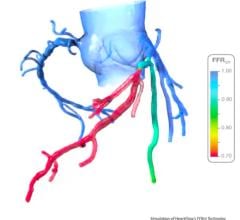
FFR is used to evaluate the severity of stenosis in arteries to determine if the lesions should be stented.
St. Jude Medical acquired its FFR technology with the purchase of Radi Medical Systems in December 2008. Volcano is the only other company that offers an FFR system that is cleared for use by the U.S. Food and Drug Administration (FDA).
In May, St. Jude acquired LightLab Imaging Inc., which is also engaged in litigation against Volcano and subsidiary Axsun Technologies Inc. in Suffolk County, Mass. LightLab developed an optical coherence tomography (OCT) intravascular imaging system, which received FDA clearance earlier this year. Volcano is also developing an OCT system and Axsun is a supplier for LightLab.
For more information: www.volcanocorp.com, www.sjm.com


 February 03, 2026
February 03, 2026