October 18, 2016 — Abbott and St. Jude Medical Inc. announced today an agreement to sell certain products from their electrophysiology (EP) and vascular closure portfolios to Terumo Corp. The transaction reflects a purchase price of about $1.12 billion and is subject to the successful completion of Abbott's acquisition of St. Jude Medical and antitrust regulatory approvals.
Read the article “Abbott to Acquire St. Jude Medical,” from April 2016.
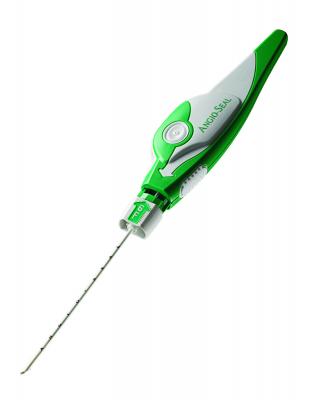
The divestiture is an all-cash transaction and will include the products globally for St. Jude Medical's Angio-Seal and Femoseal vascular closure products and Abbott's Vado Steerable Sheath. Abbott will retain its vascular closure products, which include the Perclose ProGlide Suture-Mediated Closure System, StarClose SE Vascular Closure System and Prostar XL Percutaneous Vascular Surgical System. The Angio-Seal product lines offer healthcare providers an alternative to manual compression for sealing puncture sites on patients who have undergone a catheterization procedure.
Abbott’s Vado steerable sheath is used to navigate EP ablation catheter procedures with precision to create continuous, smooth lesions. The sheath is the first design on the market that does not use pull wires for deflection, instead using connected coaxial lumens reinforced with Kevlar to provide stability in all planes. As opposed to traditional pull wire designs, this allows operators to rotate the Vado 360 degrees without torque buildup, which results in catheter whipping.
Following Abbott's acquisition of St. Jude Medical, the combined business will compete in nearly every area of the cardiovascular market and hold top positions in high-growth segments, including atrial fibrillation, structural heart and heart failure, as well as a leading position in the high-growth neuromodulation market.
Abbott expects to mitigate any impact to its adjusted earnings per share projections related to the sale of these assets to Terumo. Abbott, St. Jude Medical and Terumo are bound by the terms of an exclusivity agreement.
For more information: www.sjm.com, www.abbott.com


 November 14, 2025
November 14, 2025 









