May 4, 2012 - SMT Research & Development Ltd. (SMT) announced the addition of three key executives to its management team, including a new CEO, CFO and chief medical officer, plus two additions to its board of directors. The company also announced that its shareholders and board of directors have approved the company's name change to Keystone Heart Ltd.
Keystone Heart's new president and CEO, Mr. Shuki Porath, joined the company in November 2011 as vice president and general manager. Prior to Keystone Heart, he served as CEO at SeamVad Ltd., a medical device startup company developing disposables for vascular surgery, and was CEO at ES Vascular Ltd., developer and manufacturer of innovative open and endovascular aortic staplers for AAA procedures. Formerly, Mr. Porath served for 10 years as a senior executive at Biosense Webster, a Johnson & Johnson company. Mr. Porath holds a B.S. in electrical engineering, an M.S. in industrial engineering and a MBA – all received from the Technion, Israel Institute of Technology, Haifa, Israel.
Mr. Lior Buchman is Keystone Heart's newly appointed vice president and CFO. With more than 15 years of experience in corporate financing, M&A and business development, Mr. Buchman joins Keystone Heart, having spent the last four years as VP Finance and CFO at NiTi Surgical Solutions. Formerly, he served as VP Finance and CFO for medical device companies and VCs including Stryker GI, Sightline and Primary Ventures. Mr. Buchman is a Certified Public Accountant and holds a B.A. in accounting and economics from the University of Haifa, Israel.
M. Pauliina Margolis, M.D., Ph.D. has joined Keystone Heart as vice president and chief medical officer. Prior to Keystone Heart, Margolis served as CMO and VP Scientific Affairs at Volcano Corp., specializing in enhancing diagnosis and treatment of coronary and peripheral vascular disease. She spent 12 years at Volcano in a variety of positions. Formerly, Dr. Margolis worked with medical device startups, VCs and large companies in Silicon Valley.
Margolis has over 50 original scientific publications, several reviews and has written a number of book chapters on cardiology. She has post-doctoral training in coronary ischemia (University of Cincinnati) and is a specialist in internal medicine (University of Helsinki), interventional cardiology (University of Edinburgh), early business development and executive management (Stanford Business School).
Mr. Udi Arad of Arad Law and Mr. David Fuchs of B&P Management have also been added to Keystone Heart's Board, joining Nissim Darvish, David Bonita and Vince Burgess of OrbiMed Advisors, LLC on the board of directors.
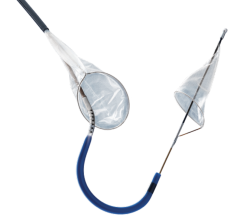
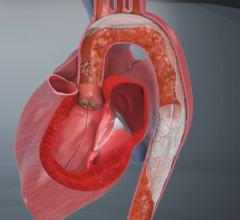
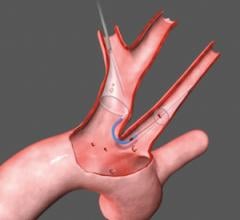
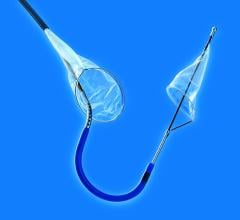
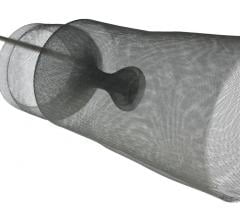
The new company name reflects its position as key in facilitating cerebral protection during left-sided procedures in the heart. Positioned at the top of the aortic arch, Keystone Heart's embolic deflection device is designed to protect all three take-offs leading to the brain. The proprietary deflection device is designed for use during TAVI (Transcatheter Aortic Valve Implantation) and other complex structural heart procedures.
"Our company mission is to help reduce the incidence of brain injury (stroke and so called "silent stroke") during TAVI and other structural heart procedures. This is reflected in both our new name and the changes in Keystone Heart's management," said Mr. Vince Burgess, chairman of the board. "We are extremely pleased to have attracted such seasoned and globally respected medical device executives to the company. I strongly believe that their addition will enable the company to achieve set goals and become a significant participant in this life-changing field."
For more information: www.keystoneheart.com
News | May 04, 2012


 April 25, 2023
April 25, 2023