January 17, 2012 – Terumo Americas Holding Inc., a U.S. subsidiary of Japan's Terumo Corp., announced it acquired Onset Medical Corp., which develops interventional procedural access sheath technology for cardiology and urology.
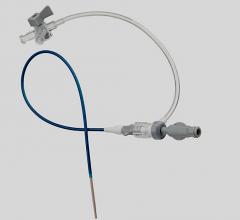
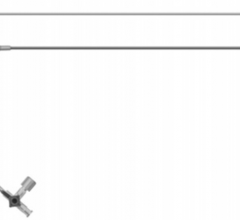
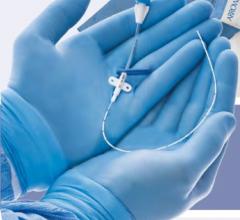
Using its proprietary controlled deployment technology (CDT) platform, Onset has developed the smallest sheath profiles with the largest internal diameters of any available access sheath to protect against unnecessary access site or arterial trauma for the patient. Onset's Associates will join with TAH subsidiary Terumo Medical Corporation as part of its Terumo Interventional Systems (TIS) business unit, where the CDT platform will complement TIS' complete product line of industry leading entry site management and lesion access technologies.
Terumo customers will have access to Onset's complete product lines. The SoloPath balloon expandable transfemoral and transseptal catheters' expandable and collapsable sheath technology allows TIS to enter the global structural heart and aneurysmal repair markets, providing an access platform for complex, large-bore procedures including transcatheter aortic valve implantation (TAVI), thoracic endovascular aortic/aneurysm repair (TVAR) and endovascular aneurysm/aortic repair (EVAR). The Pathway balloon expandable PCNL sheath and Pathway balloon expandable ureteral access sheath provide physicians with a one-step option for performing procedures such as percutaneous nephrolithotomy in the removal of large renal calculi (kidney stones).
For more information: www.terumomedical.com

 June 10, 2020
June 10, 2020