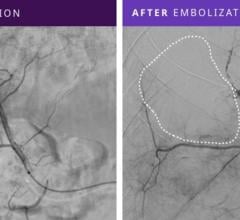
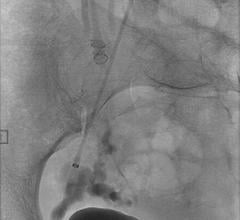
January 28, 2008 - Terumo Interventional Systems launches the AZUR Peripheral HydroCoil Embolization System for the occlusion of blood vessels, vascular malformations and aneurysms, extending its interventional device portfolio.
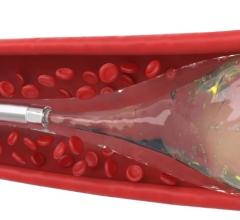
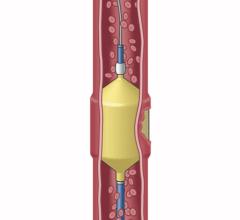
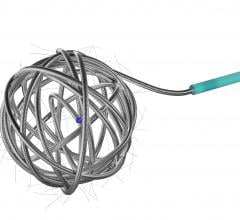
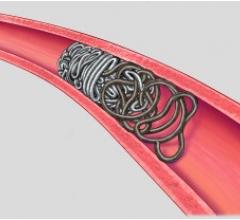
AZUR is a peripheral platinum coil embolization system with a hydrogel coating that expands when introduced into the bloodstream, designed to deliver greater filling and mechanical occlusion - all with fewer coils.
Because AZUR�s hydrogel coating undergoes limited expansion within the first three minutes and fully expands within 20 minutes, it reportedly results in five times more filling volume for the 0.018" coil and nearly and 4 times more filling volume for the 0.035" coil as well as increasing mechanical stability with fewer coils.
The AZUR peripheral embolization system offers a detachable system, designed to give physicians the ability to detach coils in less than a second and at the push of a button. With the capability to withdraw and reposition the coil until the coil is securely placed, the system minimizes the risk of non-target embolization and coil migration.
For more information: www.terumois.com


 June 05, 2025
June 05, 2025