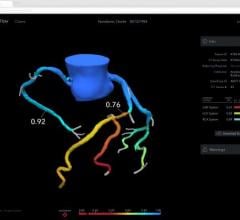
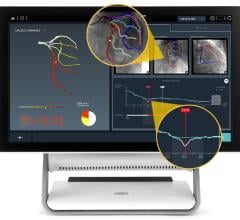
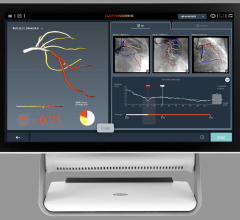
Jan. 29, 2007 — Radi Medical Systems Inc. says today it has begun the first shipment to customers of PressureWire Certus, a hydrophilic coated pressure wire designed better maneuverability, compatibility and reliability during interventional cardiology procedures.
“The PressureWire Certus certainly provides a large performance improvement over previous generations,” said Dr. Massoud Leesar, University Hospital, Louisville, KY. “The wire is very torqueable and we advanced it to difficult locations with ease."
An over-the-wire version will be available for shipment in early February, the company says.


 February 03, 2026
February 03, 2026