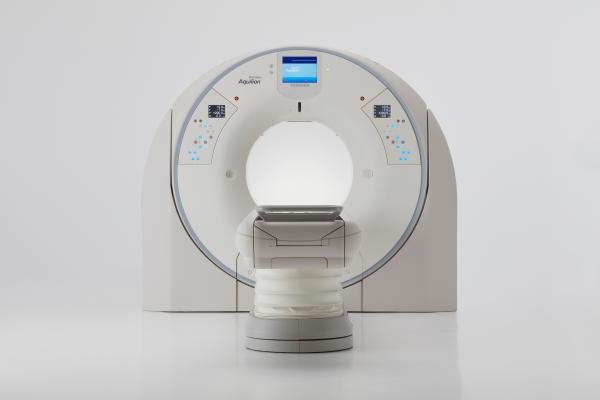
December 6, 2017 — Toshiba Medical, a Canon Group company, showcased the Aquilion Precision, what it calls the world’s first ultra-high resolution computed tomography system (UHR CT) at the 2017 Radiological Society of North America (RSNA) Annual Meeting, Nov. 26-Dec. 1 in Chicago. The system, which is pending U.S. Food and Drug Administration (FDA) 510(k) clearance, is capable of resolving anatomy as small as 150 microns, providing CT image quality with resolution typically seen only in cath labs. The UHR detector is newly designed to provide more than twice the resolution when compared with today’s CT technology, featuring an all-new detector as well as tube, gantry and reconstruction technologies.
The Aquilion Precision has the potential to help improve early detection of disease and tumor classification thanks to features that offer enhanced image detail, including:
- Dose efficiency: Detector channels that are only 0.25 mm thick, coupled with substantial improvements in scintillator quantum efficiency, detector circuitry and other DAS components, result in a dose-efficient detector with ultra-high resolution capabilities.
- Enhanced resolution: The system features the industry’s smallest Focal Spot Tube, according to Toshiba, at 0.4 mm x 0.5 mm and what it calls the industry’s first 1,024 and 2,048 Reconstruction Matrix for further increased resolution.
For more information: www.medical.toshiba.com


 February 02, 2026
February 02, 2026 









