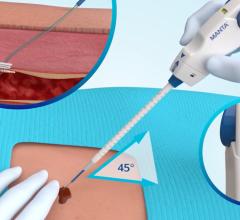
August 7, 2014 — Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval for the velox CD Vascular Closure Device. Velox CD provides a safe and predictable means for achieving immediate hemostasis following percutaneous femoral procedures. The single size velox CD is indicated for closing arteriotomies broadly ranging in size from 5 French to 8 French.
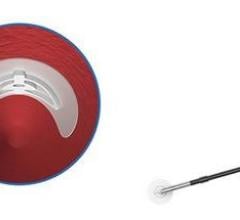
Velox CD utilizes patented mechanisms to provide an easy-to-use, single-use instrument to deliver an implant to the vessel wall, thereby avoiding the need for time-consuming manual compression and allowing for shorter patient time-to-ambulation. The implant is wholly made from a proprietary magnesium alloy designed to bioabsorb rapidly after implantation. The implant's intraluminal portion dissolves within 24 hours, while the remainder is completely resorbed within two weeks.
CE mark approval has been granted on the strength of a pivotal clinical trial in which the safety and effectiveness of the device in fully anti-coagulated patients was demonstrated.
Shing-Chiu Wong, director of cardiac catheterization laboratories at the New York Presbyterian — Weill Cornell Medical Center and a leading clinical participant in the velox CD clinical studies stated, "Velox CD is the world's first metal-based bioabsorbable closure device that is simple to use and highly predictable for femoral access site hemostasis. This innovation should prove to be a significant enhancement to the existing offerings."
For more information: www.transluminal.net

 October 07, 2025
October 07, 2025