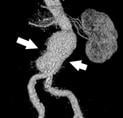
April 9, 2010 – The first patients have been enrolled in the INNOVATION trial, which will assess the safety of a new stent graft system to treat abdominal aortic aneurisms (AAA).
Current AAA stent-grafts limit the range of patients suitable for endovascular aneurism repair (EVAR). Delivery and accurate placement can also be challenging. The new Incraft stent graft is supposed to be easier to implant due to its smaller size. Currently available EVAR devices have a system profile ranging from 18 to 24 French. Incraft’s delivery system profile is 13 French.
The INNOVATION trial (multicenter, open-label, prospective, non-randomized study of the Cordis AAA stent graft system in subjects with abdominal aortic aneurysm) will enroll up to 25 patients at three sites throughout Germany.
“The ability to customize the Incraft system during the procedure is a very helpful feature for clinicians,” said professor Giovanni Torsello, chief of vascular surgery at St. Franziskus Hospital in Muenster.
The initial procedures using the device were performed by professor Dierk Scheinert, head of the department of medicine, angiology and cardiology at Park-Krankenhaus Hospital in Leipzig, who is serving as principal investigator of the INNOVATION trial. “We have been excluding a significant portion of our AAA patients, especially women, from EVAR because current stent grafts have large and bulky delivery systems, making device introduction impossible for small or diseased access vessels,” he said. “The ultra-low profile delivery system of Incraft will make EVAR a possible treatment alternative for a wider range of patients.”
“I believe the Incraft stent graft system has the potential to impact how we treat AAA patients globally.” said Takao Ohki, M.D., chairman of the department of surgery at Jikei University School of Medicine in Tokyo, who has one of the largest AAA practices in the world.
The trial is sponsored by Cordis and both investigators and sites are under contract with Cordis to perform this research. The Physician Advisory Panel members are compensated for their time by Cordis.
An estimated 27 million people worldwide have abdominal aortic aneurysms. Left untreated, all aneurysms will eventually rupture, and more than 80 percent of aneurisms that rupture result in death. In the U.S. alone, about 15,000 people die every year due to an AAA rupture.
For more information: www.cordis.com


 April 26, 2023
April 26, 2023 









