February 26, 2009 - In 2006, Americans were exposed to more than seven times as much ionizing radiation from medical procedures as was the case in the early 1980s, according to a new report on population exposure released March 3rd by the National Council on Radiation Protection and Measurements (NCRP) at its annual meeting in Bethesda, MD.
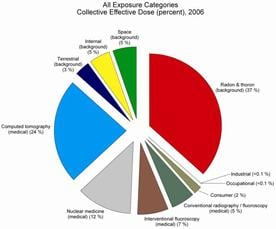
In 2006, medical exposure constituted nearly half of the total radiation exposure of the U.S. population from all sources. The increase was primarily a result of the growth in the use of medical imaging procedures, explained Dr. Kenneth R. Kase, senior vice president of NCRP and chairman of the scientific committee that produced the report.
“The increase was due mostly to the higher utilization of computed tomography (CT) and nuclear medicine. These two imaging modalities alone contributed 36 percent of the total radiation exposure and 75 percent of the medical radiation exposure of the U.S. population.” The number of CT scans and nuclear medicine procedures performed in the U.S. during 2006 was estimated to be 67 million and 18 million, respectively.
The NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, provides a complete review of all radiation exposures for 2006.
Background radiation, which in 2006 contributed fully half of the total exposure, comes from natural radiation in soil and rocks, radon gas which seeps into homes and other buildings, plus radiation from space and radiation sources that are found naturally within the human body.
Other small contributors of exposure to the U.S. population included consumer products and activities, industrial and research uses and occupational tasks.
NCRP is working with some of its partners like the American College of Radiology (ACR), World Health Organization and others to address radiation exposure resulting from the significant growth in medical imaging and to ensure that referrals for procedures like CT and nuclear medicine are based on objective, medically relevant criteria (e.g., ACR appropriateness criteria).
This year marks the 80th anniversary of NCRP’s founding and the 45th anniversary of its charter from the U.S. Congress under Public Law 88-376.
To read NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the U.S.:
http://www.ncrponline.org/PDFs/Elec_prepub_160.pdf
For more information: NCRPpublications.org


 February 02, 2026
February 02, 2026 









