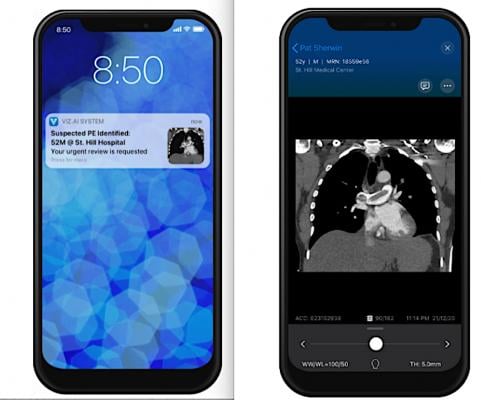
An example of Viz.AI's pulmonary embolism AI application and mobile alert to the physician on-call.
July 21, 2021 — Artificial intelligence (AI) medical imaging vendors Viz.AI and Avicenna.AI have partnered to enable intelligent care coordination and improve patient triage of patients suffering from pulmonary embolism (PE) and aortic disease. The collaboration will pave the way for faster clinical decision making and care for patients suffering from these two life threatening conditions.
Diagnosis and care coordination of patients suffering from PE and aortic disease can be challenging. Avicenna.AI’s FDA-approved algorithms for PE and Type A and Type B aortic dissection (AD), leveraged on the Viz intelligent care coordination platform, will empower multidisciplinary care teams to easily coordinate patient care by sending notifications paired with dynamic imaging and detailed patient information to each provider's desktop or mobile device as soon as a PE or an aortic dissection is detected. As part of Viz.ai’s commercial aortic module offering, access to imaging and workflows supporting the coordination of care for patients with abdominal aortic aneurysm (AAA), thoracic aortic aneurysm (TAA), rupture, stenosis and transection will be available in addition to the AD algorithm. Similarly, the PE algorithm will be available on Viz.ai’s commercial PE module.
“The addition of AI powered workflows should decrease the time from diagnosis to treatment and help to coordinate care for patients suffering from life-threatening PE and aortic disease, as it has in the treatment of ischemic stroke. It will help to facilitate faster and easier treatment decisions across health systems, and this should improve outcomes for patients,” said Dr. Richard Saxon, interventional radiologist Tri-City Medical Center.
The Viz Platform is proven to save time and improve patient outcomes. In 2020, CMS granted Viz.ai a New Technology Add-on Payment (NTAP) for the stroke module, Viz LVO, which reimburses hospitals directly. Pairing team alerts enhanced by AI with high-fidelity mobile image viewing, patient information and full-stack secure communication enables multidisciplinary teams to make faster treatment decisions for patients. The result increases access to care and improves outcomes for patients.
"We are proud to become a trusted partner of Viz.ai, who is committed to giving access to high-standard healthcare systems worldwide. Thanks to our AI-based solutions, we hope to facilitate an optimal medical response within a short time frame and improve patient care. We look forward to bringing the benefits of our pulmonary embolism and aortic dissection triage tools to the emergency room and beyond," said Cyril Di Grandi, chief executive officer of Avicenna.AI.
PE is a potentially deadly form of venous thromboembolic disease, a common cause of cardiovascular death, and is associated with multiple inherited and acquired risk factors affecting hundreds of thousands of people every year globally. PE occurs when a blood clot gets lodged in an artery in the lung, blocking blood flow to part of the lung. Similar to PE, aortic disease is potentially deadly and can require urgent surgical intervention to prevent death.
“We’re delighted to partner with Avicenna to bring intelligent care coordination to the peripheral vascular and vascular fields. This will form part of a broader Viz platform, where any patient with life threatening diseases can benefit from AI powered triage, fast mobile communication and synchronized care coordination,” said Dr. Chris Mansi, Viz.ai CEO and co-founder. “This partnership will bring the advantages of artificial intelligence to more patients and help hospitals achieve better clinical outcomes.”
Viz.ai’s clinically validated platform leverages advanced deep learning to communicate time-sensitive information to specialists who can more quickly and easily make treatment decisions for the patient. In February 2018, the U.S. Food and Drug Administration (FDA) granted a de novo clearance for Viz LVO, the first-ever computer-aided triage and notification software. Viz.ai announced its second FDA clearance for Viz CTP through the 510(k) pathway, offering healthcare providers an important tool for automated cerebral perfusion image analysis. A third FDA clearance was granted in 2020 for Viz ICH, which uses AI to automatically detect suspected intracranial hemorrhage on CT imaging. In 2020, CMS granted Viz.ai the first New Technology Add-on Payment (NTAP) for artificial intelligence software for Viz LVO.
For more information: www.avicenna.ai, www.viz.ai
For more, read the RSNA 2021 article AI Can Facilitate Automated Activation of Pulmonary Embolism Response Teams
AI Triage Solution for PE and Aortic Dissection Receives FDA Clearance and CE Mark


 September 18, 2025
September 18, 2025 









