April 29, 2008 - Nihon Kohden America Inc. said its Prefense Early Detection and Notification System received 510(k) clearance from the FDA, paving the way for its release to customers.
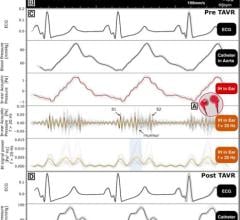
Prefense is designed to protect hospital patients from medical harm and enhance the efficiency of rapid response teams. Prefense is unique as it allows hospital patients to freely ambulate while measuring heart rate, blood pressure, oxygen saturation and respiration - four of the seven critical parameters that trigger a rapid response team.
The device aims to provide continuous monitoring and observation to reduce risk exposure among the large number of patients in Med/Surg wards, where many of the 15 million instances of medical harm occur each year.
The Prefense system consists of a new, low acuity central station interface that is simple, easy to use and cost-effective, combined with the company's NTX wireless telemetry transmitters. Future versions will also include pagers for nurses, rapid response team members and other care givers in need of notification. Prefense is the only solution that seamlessly integrates the mobility of Nihon Kohden America's NTX, the advance warning capabilities of continuous trend analysis and the proven effectiveness of Rapid Response Teams.
For more information: www.nihonkohden.com


 October 21, 2025
October 21, 2025