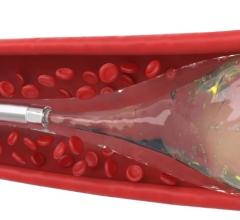
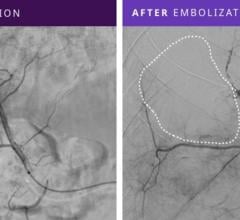
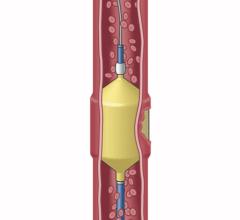
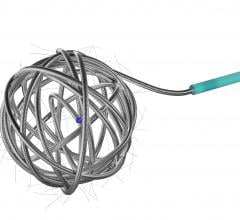
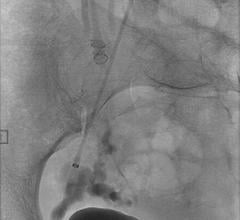
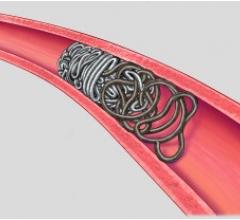
December 17, 2010 – The first truly detachable hydrogel polymer embolic device is now available nationwide. The AZUR Peripheral HydroCoil 35 platinum coil embolization system, from Terumo Interventional Systems, is designed for treating high-flow and challenging blood vessels, vascular malformations and aneurysms.
The 0.035-inch coils provide greater filling capacity, packing density and mechanical occlusion.
The hydrogel coating undergoes limited expansion within the first three minutes, and fully expands within 20 minutes, offering nearly four times more filling volume than a platinum coil of the same size. It deploys rapidly - taking less than a second - while offering physicians the flexibility to position and observe the behavior of the coil in the vasculature prior to detachment. It can also be retracted and repositioned until it is securely placed, reducing the risk of non-target embolization and coil migration.
In addition, the system is the only hydrogel embolic indicated for peripheral applications that is detachable and compatible with 0.038-inch lumen catheters. The microporous expandable hydrogel is biologically inert and provides scaffolding for natural tissue proliferation.
"In our experience, the AZUR Detachable 35 is an excellent embolization system that is very capable of treating higher capacity lesions with fewer coils, while effectively minimizing risk of coil migration,” said Craig Greben, M.D., chief, division of vascular and interventional radiology, North Shore University Hospital, Manhasset, N.Y. “The larger coil size is highly deliverable and provides us with added confidence in treating high-blood flow areas or particularly challenging anatomies.”
The device is intended to reduce or block the rate of blood flow in vessels of the peripheral vasculature. It is designed for use in the interventional radiologic management of arteriovenous malformations, arteriovenous fistulae, aneurysms and other lesions of the peripheral vasculature.
For more information: www.terumois.com


 June 05, 2025
June 05, 2025