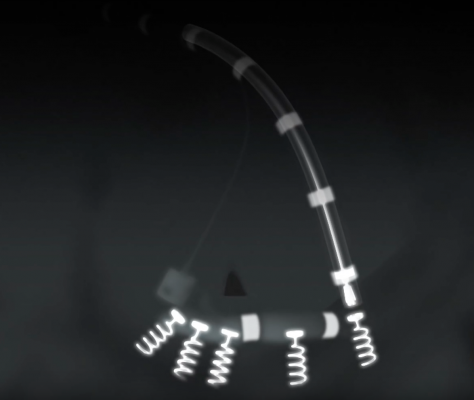
An angiography image showing the catheter-based implantation of the Cardioband annuloplasty ring using corkscrew shaped anchors.
June 13, 2018 — Henry Ford Hospital is one of 17 U.S. trial sites using a catheter-based procedure approved in Europe to repair a leaky mitral heart valve.
Henry Ford Hospital’s Center for Structural Heart Disease, led by cardiologist William W. O’Neill, M.D., performed the first Cardioband procedure in Michigan on May 31. The hospital is the only system in Michigan involved in the U.S. trial and one of a couple in the midwestern United States. The Cardioband procedure was approved for use in Europe in September 2015.
Watch a video demonstrating how to implant the Cardioband device
“At Henry Ford Hospital, we’ve been working on the mitral valve for five years while many programs are in their infancy,” said O’Neill, who was the first cardiologist in the United States to perform transcatheter mitral valve replacement (TMVR) with a valve typically used for transcathether aortic valve replacement (TAVR). “We feel this is a vital addition to our options for patients with mitral valve issues. We’ve seen the great outcomes in Europe and are very pleased to participate in the U.S. trials.”
More than 300 transcatheter mitral valve repairs and replacements have been performed at Henry Ford Hospital since the center’s transcatheter mitral program began in 2013.
Cardioband is an alternative to surgical mitral valve repairs doctors have been doing for years for patients with functional mitral valve regurgitation. In patients with the condition, the mitral valve leaks as blood passes between the left atrium and the left ventricle, most often because of deformity in the valve circumference. This can be due to damage to the left ventricle from a prior heart attack or cardiomyopathy.
The most commonly performed treatment for functional mitral valve regurgitation is annuloplasty, an open-chest surgical approach that requires weeks of recuperation. The Cardioband procedure is done strictly through the catheter as the patient’s heart continues to beat normally. The Cardioband is delivered through a small incision in the groin area, into a catheter that passes up through the femoral vein and into the heart. Using a control device that remains outside the body, cardiologists deploy a series of small springs and anchor them along the edge of the mitral valve. The cardiologist then cinches the opening of the valve to near its original shape and size, allowing the leaflets to close more efficiently.
The entire process is guided by 3-D transesophageal echocardiography (TEE), led by cardiologist Dee Dee Wang, M.D., director of imaging for the Center for Structural Heart Disease and medical director of 3-D printing for the Henry Ford Innovations Institute. Using an endoscope passed through the mouth and into the esophagus, ultrasound waves create images of the heart and passageways.
“The 3-D imaging played live on a screen provides a roadmap for guided implementation of each portion of the band,” Wang said. “This ensures patient safety and optimal outcomes.”
Most patients will see immediate improvement in their breathing and other side-effects of mitral valve regurgitation. Patients will typically go home within a couple of days of the procedure, said Henry Ford cardiologist Marvin Eng, M.D., who performed the May 31 Cardioband procedure with O’Neill.
“I think it’s a wonderful opportunity because these patients don’t usually have alternatives,” said Eng, director of research for the Center of Structural Heart Disease and the center’s fellowship director. “We are glad to add another option to our suite of procedures offered at Henry Ford to treat the mitral valve.”
The ACTIVE Trial is a prospective, randomized, multicenter trial registered at ClinicalTrials.gov. Patients with clinically significant functional mitral regurgitation will be randomized 2:1 to receive either medical therapy or transcatheter mitral valve repair with the Cardioband plus medical therapy. Patients will be seen for follow-up visits at discharge, 30 days, six months and annually through five years.
Researchers estimate the prevalence of valvular heart disease at 2.5 percent in the U.S. population, sharply increasing after age 65 as valves age. The proportion of valvular surgeries has increased in recent years and is estimated to account for 20 percent of all cardiac surgeries.
For more information: www.henryford.com
Additional articles and videos on Henry Ford Hospital
Related Mitral Valve Content
Transcatheter Annuloplasty For Repair Versus Replacement in Functional Mitral Regurgitation
Edwards Granted CE Mark For First Transcatheter Tricuspid Therapy
VIDEO: Transcatheter Mitral Valve Implantation in Practice and Technologies in Development
VIDEO: Update of Mitral Valve Repair and Replacement Technologies


 November 14, 2025
November 14, 2025 









