February 24, 2023 — Abiomed, Inc., part of Johnson & Johnson MedTech[1], announced that results from a study on Impella-supported high-risk percutaneous coronary intervention (HRPCI) patients will be presented during the upcoming Cardiovascular Research Technologies (CRT) 2023 Conference in Washington, DC from Feb. 25-28. Abiomed’s booth (#503) and Saturday’s Power Break lunch symposium will feature the latest Impella technologies. Several Live Cases with physicians using Impella technology will be broadcast throughout the conference to attendees.
Oral Presentation
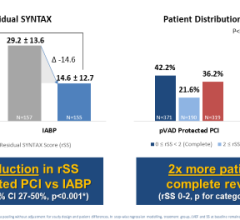
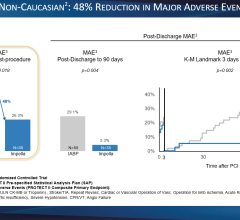
Named one of the top oral presentations at CRT 2023, Arsalan Abu-Much, MD, from the Cardiovascular Research Foundation, will highlight results from a PROTECT III analysis of 1,237 non-cardiogenic shock patients who received an Impella supported HRPCI.
- Title: Impella Utilization In High-risk Percutaneous Coronary Intervention Mitigates The Risks Of Procedural And Clinical Adverse Events Independent Of Left Ventricular Ejection Fraction: The Protect III Study
- Date/Time: Sunday, Feb. 26, 2:30-2:40 pm ET, Kent Theater
Symposium – High Risk, High Reward – Modern Impella Utilization
On Saturday, Feb. 25 at 1 pm ET, Abiomed is sponsoring a lunch symposium focused on Impella utilization during HRPCI. Four physicians, listed below, will discuss how to identify appropriate patients for HRPCI with Impella support as well as optimizing outcomes for these patients. Two Impella HRPCI cases will also be featured, including one case utilizing Impella ECP, the world’s smallest heart pump at 9 Fr.
- William W. O'Neill, MD, FACC, MSCAI – Henry Ford Health Systems
- Katherine Kunkel, MD – Piedmont Heart Institute
- Amir Kaki, MD, FACC, FSCAI – Ascension St John Hospital
- Michael C. Kim, MD – Lenox Hill Hospital, Northwell Health
Abiomed Technology Highlights
At the Abiomed booth (#503), visitors will have the opportunity to learn more about a recent labeling change for Impella CP with SmartAssist and get hands-on demonstrations with Abiomed’s latest technologies. The U.S. Food and Drug Administration (FDA) recently validated the best practice of placing Impella pre-PCI in cardiogenic shock patients. The FDA’s decision is based on multiple studies and publications and a recently published AHA Scientific Statement, which says physicians should consider Impella prior to initiation of PCI in patients presenting with signs and symptoms of cardiogenic shock. The following technologies will be featured at the booth:
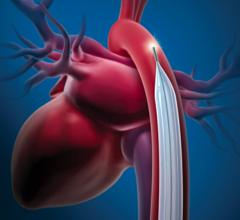
- Impella RP Flex with SmartAssist is implanted percutaneously through the internal jugular vein, which provides the option for patient mobility while on support and is designed to be easy to implant, with a flexible canula that is advanced over an extra supportive guidewire.
- Impella Low Profile Sheath has smaller size and technological advancements that facilitates easier Impella insertion and removal, reduces procedural steps and helps improve outcomes.
- 9th generation of Impella CP with SmartAssist has a new tapered, more flexible pigtail that enables easier insertion and is compatible with heparin-free purge, sodium bicarbonate, to help with anticoagulation management.
For more information: www.abiomed.com
Related Impella Content:
VIDEO: Demonstration of Abiomed Impella ECP 9 French Transcatheter Ventricular Assist Device
VIDEO: Abiomed Highlights Trends and New Technology in Hemodynamic Support
FDA Approves First-in-Human Trial of Impella ECP World’s Smallest Heart Pump
VIDEO: Demonstration of the Impella Percutaneous Hemodynamic Support Device
VIDEO: Tufts Uses a Hemodynamic Support Algorithm to Determine What Devices to Use — Interview with Navin Kapur, M.D.
Photo Gallery of the Abiomed Impella Production Line
VIDEO: Door-to-Unloading (DTU) Trial May Change STEMI Care — Interview with Navin Kapur, M.D.
VIDEO: Hemodynamic Support Protocols at Henry Ford Hospital — Interview with William O'Neill, M.D.

 August 14, 2023
August 14, 2023