Photo: Abbott
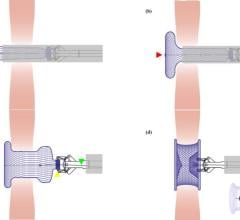
Dec. 18, 2025 — Abbott has received U.S. Food and Drug Administration (FDA) clearance and CE Mark for its Amplatzer Piccolo Delivery System, which is used with the company's Amplatzer Piccolo Occluder. The new delivery system is designed specifically to treat premature babies (some weighing as little as two pounds) with a hole in the heart known as a patent ductus arteriosus (PDA).
A PDA is an opening between two blood vessels in an infant's heart that fails to close as it should following birth. Before a baby is born, this channel allows blood to bypass the not-yet-functioning lungs because the fetus gets oxygen-rich blood from the mother. If the hole remains open after birth, it changes the flow pattern so additional blood is directed to the lungs, making it difficult for babies to breathe normally.
"Abbott's new Amplatzer Piccolo Delivery System is a transformative step forward in how we treat PDA in premature infants," said Evan Zahn, M.D., professor of cardiology and pediatrics and director of the Guerin Family Congenital Heart Program at Cedars-Sinai Medical Center in Los Angeles, Calif. "The new delivery system simplifies the implant procedure because only one catheter is needed instead of multiple, and a shorter and softer design allows for more precise device positioning in these tiny babies. Doctors can treat this group with more confidence, reducing the risk of adverse events and improving the long-term outlook for this uniquely vulnerable patient population."
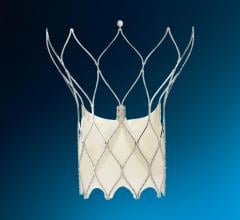
The Amplatzer Piccolo Occluder is smaller than a pea and is the world's first and only minimally invasive, transcatheter treatment approved to close a PDA in premature infants with this common congenital heart defect. The Amplatzer Piccolo device is inserted through a small incision in the infant's leg and guided through vessels to the heart using the Amplatzer Piccolo Delivery System, where it is placed to seal the opening in the heart. The Amplatzer Piccolo Occluder received FDA approval and CE Mark in 2019.
"We designed the Amplatzer Piccolo Delivery System based on feedback from leading physicians across the world to make PDA closure procedures even safer and easier," said Sandra Lesenfants, senior vice president of Abbott's structural heart business. "With the Amplatzer Piccolo Occluder, which is the world's smallest heart device, and now with the new delivery system to complement it, we're continuing to advance how we meet the needs of our tiniest patients with structural heart disease."
Abbott's portfolio of pediatric heart therapies also includes the world's smallest mechanical heart valve, the Masters HP 15mm, and the HeartMate 3 heart pump, which is approved for pediatric patients. Abbott is continuing to develop lifesaving pediatric devices that have an immediate impact with long-term benefits, reduce the risks of life-threatening complications and allow physicians to confidently treat the youngest and tiniest patients.
Learn more at www.abbott.com.
- For U.S. important safety information on the Amplatzer Piccolo Delivery System, visit https://abbo.tt/PDS_ISI.
- For U.S. important safety information on the Amplatzer Piccolo Occluder, visit https://abbo.tt/PiccoloISI.
- For U.S. Important Safety Information about the Masters HP 15mm valve, visit https://abbo.tt/MastersISI.
- For U.S. Important Safety Information about the HeartMate 3, visit https://cardiovascular.abbott/hm3isw.
1 emedicine. April 1, 2025. Patent Ductus Arteriosus. https://emedicine.medscape.com/article/891096-overview.


 June 20, 2024
June 20, 2024