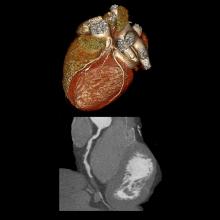
The American College of Cardiology and the American Heart Association today released a revised guideline for minimizing the risk of cardiovascular complications around the time of noncardiac surgery after conducting a new review of research and reevaluating data from a controversial trial about the use of beta-blockers before surgery.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now