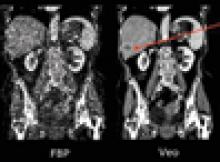
There were three key trends in X-ray vascular imaging systems made evident from new products showcased at the Radiological Society of North America (RSNA) annual meeting in late 2012. These include new hardware and software to lower ionizing radiation doses and improve image quality, better dose tracking software, and enhanced maneuverability for improved patient access and use in the growing hybrid OR market. As interventional procedures become more complex, imaging times have increased, raising concerns that are now being addressed by vendors.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now