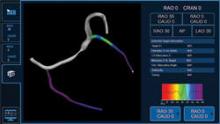
Researchers from Boston University School of Medicine (BUSM) have shown that using magnetic resonance imaging (MRI) to measure blood flow over atherosclerotic plaques could help identify plaques at risk for thrombosis. The findings, which appear in the March issue of Circulation Cardiovascular Imaging, offer a non-invasive application in the diagnosis and treatment of patients with atherosclerosis.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now