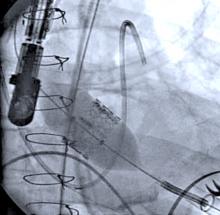
W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new large diameter 35 mm trunk-ipsilateral leg and 36 mm aortic extender components, as well as the lower profile 31 mm diameter trunk-ipsilateral leg and 32 mm aortic extender components of the Gore Excluder AAA Endoprosthesis. The new components provide physicians with a proven and durable endovascular option to treat abdominal aortic aneurysms (AAAs).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now