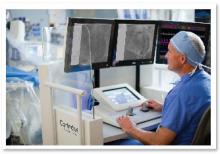
The Detroit Medical Center (DMC) announced that a team of heart specialists at its Cardiovascular Institute (CVI) has successfully conducted the Midwest's first-ever "robotic-assisted" coronary revascularization to relieve heart artery blockages. The successful implementation of the pioneering new treatment procedure — unique in Michigan and so far performed at only three institutions in the United States — means that DMC heart care patients now have access to the world's most advanced treatment method for relieving blockages in heart arteries, said CVI President Theodore L. Schreiber, M.D.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now