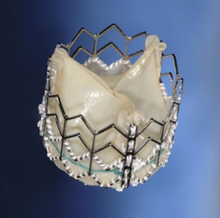
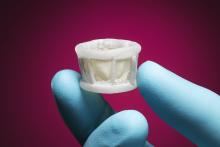
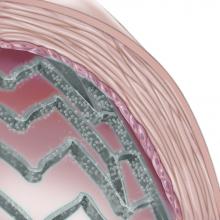
Edwards Lifesciences Corporation announced three-year results of a pivotal clinical study of inoperable patients with severe aortic stenosis treated with the Edwards Sapien transcatheter heart valve. The data from The PARTNER Trial (Cohort B) demonstrated a sustained and increasing survival benefit for Sapien valve patients at three years, as well as significantly less time spent in the hospital. The new results were presented at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now