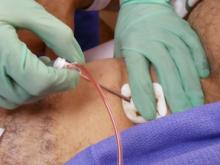
July 27, 2012 — University of Michigan heart researchers are shedding light on a safer method for steadying an abnormal heart rhythm that prevents collateral damage to healthy cells.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now