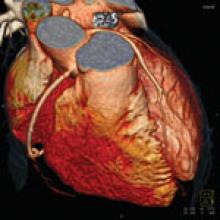
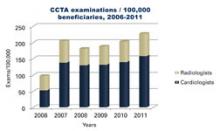
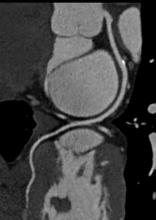
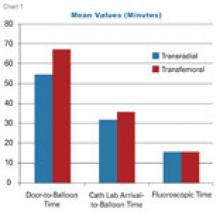
Each year, I find it interesting that some “new” technology being introduced by a vendor is actually an old, recycled idea.
A Siemens information technology executive showed me a video from the late 1970s of him as a young man demonstrating a futuristic computer system. It stored radiology images and reports electronically to eliminate the need for paper medical records. It was an idea 20 years ahead of its time, when the concept of a picture archiving and communications system (PACS) was very foreign.