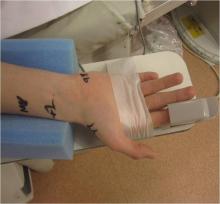
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today announced the successful deployment of a 3x100 mm Proteus device for treating peripheral artery disease (PAD) in below-the-knee (BTK) vasculature at Community Hospital in Munster, Ind.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now