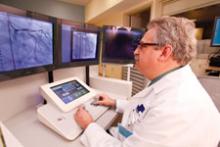
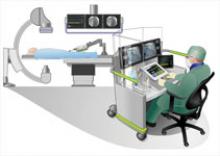
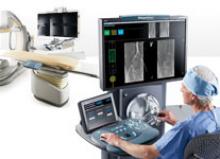
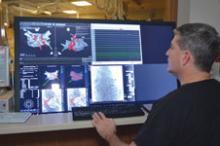
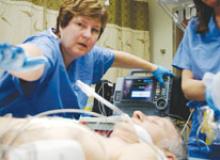
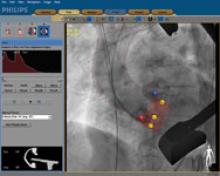
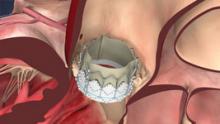
Interest has exploded over the past year in robotic cath lab guidance technology since Corindus began its U.S. Food and Drug Administration (FDA) pivotal trial. The Corindus CorPath 200 robotic-assisted percutaneous coronary intervention (PCI) system has been designed to provide precise, robotic-assisted placement of coronary guide wires and stent/balloon catheters in PCI procedures.
If you enjoy this content, please share it with a colleague
- Read more about Robots in the Cath Lab
- Log in or register to post comments